The polarity of a molecule depends on the geometric sum of the individual dipoles. Explanation: Let's consider two molecules: CX4(X =Cl), and CH Cl3. Now carbon tetrachloride
Carbon tetrachloride
Carbon tetrachloride, also known by many other names is an organic compound with the chemical formula CCl₄. It is a colourless liquid with a "sweet" smell that can be detected at low levels. It has practically no flammability at lower temperatures. It was formerly widely used in fire extinguishers, …
What factors determine the polarity of a molecule?
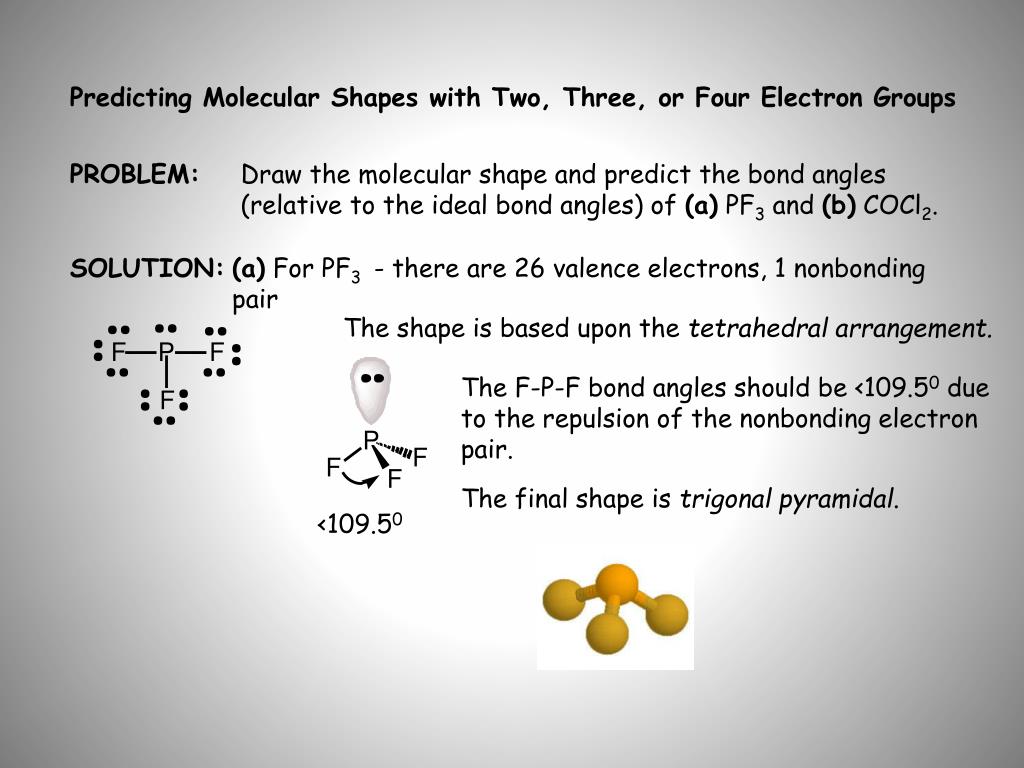
Method 1 Method 1 of 3: Drawing Lewis Dot Structures Download Article
- Write the symbols for all atoms in the molecule. Atomic symbols for atoms can be found on the periodic table.
- Find the central atom. The central atom is the atom that all (or at least most) of the other atoms are bonded to.
- Add all of the bonds. ...
- Include unbound electrons. ...
- Look for dipoles. ...
How does VSEPR theory explain molecular shape?
- VSEPR Rules:
- Identify the central atom.
- Count its valence electrons.
- Add one electron for each bonding atom.
- Add or subtract electrons for charge (see Top Tip)
- Divide the total of these by 2 to find the total.
- number of electron pairs.
- Use this number to predict the shape.
Do polar bonds affect molecular shape?
The symmetric shape and the fact that the polarities of the bonds are exactly the same means that the polarities of the bonds cancel each other out, leaving the molecule as a whole nonpolar. Many molecules are nonpolar, but have polar bonds.
How do I determine the molecular shape of a molecule?
- Write down the central atom and its outer (or valence) shell electrons.
- Pair each valence electron with an electron from the bonding atom
- To determine the “lone pairs” provided by the central atom divide the number of remaining electrons by two— because each “lone pair” contains two electrons.
How does molecular geometry determine the polarity of a molecule?
In a diatomic molecule (X2 or XY), there is only one bond, and the polarity of that bond determines the polarity of the molecule: if the bond is polar, the molecule is polar, and if the bond is nonpolar, the molecule is nonpolar.
What factors affect the polarity of a molecule?
The shape of a molecule and the polarity of its bonds determine the OVERALL POLARITY of that molecule. A molecule that contains polar bonds, might not have any overall polarity, depending upon its shape.
How does molecular shape affect polarity apex?
1 Answer. The shape of the molecule will determine the direction of each of the individual bond dipoles, and thus, will always play a role in determining the polarity of the molecule as a whole.
What determines the polarity of a bond?
The difference in electronegativity between two atoms determines how polar a bond will be. In a diatomic molecule with two identical atoms, there is no difference in electronegativity, so the bond is nonpolar or pure covalent.
How do polar covalent bonds work?
As discussed previously, polar covalent bonds connect two atoms with differing electronegativities, leaving one atom with a partial positive charge (δ+) and the other atom with a partial negative charge (δ–), as the electrons are pulled toward the more electronegative atom. This separation of charge gives rise to a bond dipole moment. The magnitude of a bond dipole moment is represented by the Greek letter mu ( µ) and is given by the formula shown here, where Q is the magnitude of the partial charges (determined by the electronegativity difference) and r is the distance between the charges:
What is the molecular structure of a polyatomic ion?
When a molecule or polyatomic ion has only one central atom, the molecular structure completely describes the shape of the molecule. Larger molecules do not have a single central atom, but are connected by a chain of interior atoms that each possess a “local” geometry. The way these local structures are oriented with respect to each other also influences the molecular shape, but such considerations are largely beyond the scope of this introductory discussion. For our purposes, we will only focus on determining the local structures.
Is electron-pair geometry the same as molecular structure?
It is important to note that electron-pair geometry around a central atom is not the same thing as its molecular structure. The electron-pair geometries shown in [link] describe all regions where electrons are located, bonds as well as lone pairs. Molecular structure describes the location of the atoms, not the electrons.
What determines the molecular polarity of a diatomic molecule?
For diatomic molecules, there is only one bond, so its bond dipole moment determines the molecular polarity. Homonuclear diatomic molecules such as Br 2 and N 2 have no difference in electronegativity, so their dipole moment is zero. For heteronuclear molecules such as CO, there is a small dipole moment.
When are molecular structures identical to electron-pair geometries?
The molecular structures are identical to the electron-pair geometries when there are no lone pairs present (first column). For a particular number of electron pairs (row), the molecular structures for one or more lone pairs are determined based on modifications of the corresponding electron-pair geometry.
How do polar covalent bonds work?
As discussed previously, polar covalent bonds connect two atoms with differing electronegativities, leaving one atom with a partial positive charge (δ+) and the other atom with a partial negative charge (δ–), as the electrons are pulled toward the more electronegative atom. This separation of charge gives rise to a bond dipole moment. The magnitude of a bond dipole moment is represented by the Greek letter mu ( µ) and is given by the formula shown here, where Q is the magnitude of the partial charges (determined by the electronegativity difference) and r is the distance between the charges:
How many regions of electron density are there in a molecule?
Two regions of electron density around a central atom in a molecule form a linear geometry; three regions form a trigonal planar geometry; four regions form a tetrahedral geometry; five regions form a trigonal bipyramidal geometry; and six regions form an octahedral geometry. Figure 3.
What is the VSEPR theory?
VSEPR theory predicts the arrangement of electron pairs around each central atom and, usually, the correct arrangement of atoms in a molecule. We should understand, however, that the theory only considers electron-pair repulsions.
How is the dipole moment determined?
For a molecule, the overall dipole moment is determined by both the individual bond moments and how these dipoles are arranged in the molecular structure.
What is the name of the structure that includes only the placement of the atoms in the molecule?
The structure that includes only the placement of the atoms in the molecule is called the molecular structure .
Answer
As discussed previously, polar covalent bonds connect two atoms with differing electronegativities, leaving one atom with a partial positive charge (δ+) and the other atom with a partial negative charge (δ–), as the electrons are pulled toward the more electronegative atom. This separation of charge gives rise to a bond dipole moment.
New questions in English
Write a short poem about any topic. Set the tone and the mood. Then, recite your poem to your parents. Afterwards, allow them to guess the one and moo …