* The reduction of a carbonyl group by LiAlH 4 is initiated by the attack of nucleophilic hydride ion on the carbonyl carbon to give a tetrahedral intermediate. * LiAlH 4 is a nucleophilic reducing agent since the hydride transfer to the carbonyl carbon occurs prior to the coordination to the carbonyl oxygen.
Why is LiAlH4 a good reducing agent?
LiALH4 is one of them most powerful reducing agents efficiently working for any carbonyl and some other functional groups as well. This high reactivity of the hydride ion in LiAlH4 makes it incompatible with protic solvents.
What is the action of LiAlH4 on alkyl halide?
Lithium Aluminium Hydride (LiAlH4) can reduce 1°& 2° alkyl halide. When reacted with 3° alkyl halide elimination takes place. In presence of AlCl3 it can reduce alkynes and benzylic alkynes to alkenes. It can also do following reductions:-
Why is NaBH4 used in carbonyl reduction instead of LiAlH4?
moreover LiAlH4 reacts violently with water so it is not useful for the water soluble carbonyl compounds..NaBH4 on the other hand is stable in water and is suitable for water soluble carbonyl compounds reduction. Plastic surgeon shares one weird way to fill in wrinkles at home.
What is the meaning of LiAlH4 in chemistry?
Source: LITHIUM ALUMINIUM HYDRIDE | LIALH4 | LAH | REDUCTION | MECHANISM. More information: * Lithium aluminium hydride, LiAlH4, also abbreviated as LAH, is a reducing agent commonly employed in modern organic synthesis. * It is a nucleophilic reducing agent, best used to reduce polar multiple bonds like C=O.
How does LiAlH4 reduce carboxylic acids?
LiAlH4 produces H−. Since H− is a strong base it should immediately abstract a proton from the carboxylic acid to give the corresponding carboxylate ion (just like in the reaction of carboxylic acids with Grignard reagents), instead of undergoing nucleophilic addition to give the alcohol.
Why is LiAlH4 a reducing agent?
Because aluminium is less electronegative than boron, the Al-H bond in LiAlH4 is more polar, thereby, making LiAlH4 a stronger reducing agent. Addition of a hydride anion (H:–) to an aldehyde or ketone gives an alkoxide anion, which on protonation yields the corresponding alcohol.
How are amides reduced with LiAlH4 mechanism?
The Mechanism of Amide Reduction by LiAlH Primary and secondary amides have a proton connected to the nitrogen that is acidic enough to be attacked by the hydride. This step occurs before the nucleophilic addition of the hydride ion: After the reaction with AlH3, the C=O.
How does LiAlH4 reduce esters?
Ch20: Reduction of Esters using LiAlH4 to 1o alcohols. Carboxylic esters are reduced give 2 alcohols, one from the alcohol portion of the ester and a 1o alcohol from the reduction of the carboxylate portion. Esters are less reactive towards Nu than aldehydes or ketones.
Is LiAlH4 a good oxidizing or reducing agent?
Chromate (LiAlH4) is a strong oxidizing agent; it oxidizes primary alcohols all the way to carboxylic acids, and secondary alcohols to ketones. Cannot be oxidized further.
Why is LiAlH4 a better reducing agent than NaBH4?
But LiAlH4 is a very strong reducing agent than NaBH4 because the Al-H bond in the LiAlH4 is weaker than the B-H bond in NaBH4. This makes the Al-H bond less stable. The reason for this is the low electronegativity of Aluminum compared to Boron.
Does LiAlH4 reduce amide to amine?
Description: Amides can be reduced to amines with a strong reducing agent like lithium aluminum hydride (LiAlH4).
What is the role of LiAlH4 in organic synthesis?
Most widely used mixed hydride in organic synthesis is LiAlH4 . It is called lithium aluminum hydride. It is a powerful reducing agent and reduces carboxylic acids to alcohols.
How are amides reduced to amines?
Amides are reduced to the corresponding amines by reaction with either metal hydrides, such as lithium aluminum hydride, or by catalytic hydrogenation. This reduction is an important process for the preparative synthesis of amines. Lactames, too, can be reduced to the corresponding pyrrolidine derivatives.
What happened when reduction of carboxylic acid with LiAlH4?
Carboxylic acids can be converted to 1o alcohols using Lithium aluminum hydride (LiAlH4).
What happens when LiAlH4 reacts with an ester?
Esters can be converted to 1o alcohols using LiAlH4, while sodium borohydride (NaBH4) is not a strong enough reducing agent to perform this reaction.
How does LiAlH4 produce nascent hydrogen?
LAH violently reacts with water, including atmospheric moisture, to liberate dihydrogen gas. The reaction proceeds according to the following idealized equation: LiAlH4 + 4 H2O → LiOH + Al(OH)3 + 4 H. This reaction provides a useful method to generate hydrogen in the laboratory.
How does LiAlH4 reduce nitriles?
Mechanism of Reduction of nitriles to primary amines by LiAlH4: Initially, the polar CN bond is added with LAH such that the negatively charged hydride makes bond with carbon. It is followed by subsequent transfer of hydride from AlH 3- group. Final protic workup generates amine group.
Which amines are reduced to primary amines by LiAlH 4?
4) The nitriles are reduced to primary amines by LiAlH 4 .
How is lithium hydride quenched?
During the workup, the reaction mixture is initially chilled in an ice bath and then the Lithium aluminium hydride is quenched by careful and very slow addition of ethyl acetate followed by the addition of methanol and then cold water.
What is the mechanism of reduction of amides to amines?
Mechanism of Reduction of Amides to amines: Amides are converted to amines. The LAH reduction mechanism is slightly different from that depicted for esters. In iminium ion is formed during the reaction since nitrogen atom is relatively a good donor than oxygen atom.
Which element reduces carboxylic acids, esters, and acid halides to corresponding primary alcohols?
2) The carboxylic acids, esters and acid halides are reduced to corresponding primary alcohols by Lithium aluminium hydride.
Which element reduces haloalkanes to corresponding hydrocarbons?
7) The haloalkanes and haloarenes are reduced to corresponding hydrocarbons by Lithium aluminium hydride.
Is methyl alcohol good for LAH?
Hence methyl alcohol is used in the quenching of LAH during workup. It is better to quench in cold conditions.
What is LiAiH4 used for?
Lithium aluminium hydride (LiAIH 4) is a nucleophilic reducing agent, and used to reduce polar multiple bonds like >C=O, -CN bond because it is hydride (H-) donor. And it is simply abbreviated as LAH. LIAIH4 can reduce: (1) Aldehydes to primary alcohols,
Does LiAH4 reduce nitriles?
LiAH4 can reduce Aldehydes, ketones, carboxylic acids, ester, acid chlorides, acids anhydrides, acid amides, Nitro Compounds and nitriles and isonitriles without attacking (Isolated non polar bonds , like -C=C- ) double bonds present in the Compounds. The only exception in this case alpha- Beta unsaturated compounds containing phenyl group in the Beta position, in this case LiAH4 also attack on double bond.
What can LiAlH4 convert to?
LiAlH4 can convert aldehydes, ketones, esters, and carboxylic acids all to alcohols in the blink of an eye.
What is the effect of LiAlH4 on the C-C bond?
LiAlH4 reduces a C-C double bond which is in conjugation i.e. resonance.
What is the electronegativity of NaBH4?
The atom holding on to the hydrides should be examined here. In the case of NaBH4 we have boron with an electronegativity of around 2.0 where as with LiAlH4 we have aluminum with an electronegativity around 1.6. Remember, the more electronegative an atom is, the less likely it will be to share or give up electrons. A hydride (H-) is just that, extra electrons (one to be exact) on a hydrogen. So if the hydride is bound to boron, it is less likely to be released for organic transformation when compared to aluminum (because boron will hold on to electrons, and thus the hydride, better).
What is the reducing agent used in organic synthesis?
Lithium aluminium hydride, LiAlH4, also abbreviated as LAH, is a reducing agent commonly employed in modern organic synthesis. * It is a nucleophilic reducing agent, best used to reduce polar multiple bonds like C=O.
Which is better, lithium or sodium?
This means that the initial activation of the carbonyl in a reduction by the metal happens more effectively with lithium. Another way of saying this is that Lithium (due to being more electropositive than sodium), is a better Lewis acid, and therefore is able to activate the carbonyl more effectively compared to sodium.
Does LiAlH4 reduce alkyl halide?
Lithium Aluminium Hydride (LiAlH4) can reduce 1° & 2 ° alkyl halide. When reacted with 3° alkyl halide elimination takes place. In presence of AlCl3 it can reduce alkynes and benzylic alkynes to alkenes. It can also do following reductions:-
Is LiAlH4 better than NaBH4?
There are two factors here in play, when trying to figure out why LiAlH4 is a better reducing agent than NaBH4. One can look both at the metal and the hydride source. First, let's look at the hydride source. A l H 4 − is a stronger hydride source than B H 4 −. This is because aluminum is less electronegative than boron, and thus the electron density is shifted more towards the hydrogen in A l H 4 − compared to B H 4 −. This makes the aluminum hydride a much better hydride donor (more electron density closer to the hydrogen, because ultimately a transfer of a H − occurs!).
What is LiAlH4 used for?
Lithium Aluminium Hydride (LiAlH4) is a nucleophilic reducing agent, best used to reduce Carboxylic esters to give 2 alcohols, one from the alcohol portion of the ester and a 1o alcohol from the reduction of the carboxylate portion.
What is the reducing agent used to reduce carboxylic esters?
Compounds containing carbonyl groups are reduced. Lithium Aluminium Hydride (LiAlH4) is a nucleophilic reducing agent, best used to reduce Carboxylic esters to give 2 alcohols, one from the alcohol portion of the ester and a 1o alcohol from the reduction of the carboxylate portion.
Is LiAlH4 a reducing agent?
Lithium aluminium hydride, LiAlH4, also abbreviated as LAH, is a reducing agent commonly employed in modern organic synth esis. * It is a nucleophilic reducing agent, best used to reduce polar multiple bonds like C=O.
Is sodium borohydride a good reducing agent?
Sodium borohydride is a good reducing agent. Although not as powerful as lithium aluminum hydride (LiAlH4), it is very effective for the reduction of aldehydes and ketones to alcohols. By itself, it will generally not reduce esters, carboxylic acids, or amides (although it will reduce acyl chlorides to alcohols).
Is lithium oxidized?
Since the reducing agent is losing electrons, it is said to have been oxidized. Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound used as a reducing agent in organic synthesis, especially for the reduction of esters, carboxylic acids, and amides.
Do ethers react with acid?
They don't react. Ethers are stable compounds and only react with acidic or electron deficient molecules. For example, they can undergo Acidic hydrolysis to give alcohols. NaBH4 is a reducing agent and it can reduce Aldehydes, ketones, acid halides and alkyl halides only. 1.2K views.
What can LiAlH4 convert to?
LiAlH4 can convert aldehydes, ketones, esters, and carboxylic acids all to alcohols in the blink of an eye.
What are some examples of LAH reductions?
An example from Breaking Bad might be the contemperaneous reduction of the nitro aldol condensate of dimethoxy benzaldehyde with nitromethane. This LAH reduction would, at the same time, hydrogenate an olefin and reduce an nitro group to an amine.
What is the electronegativity of NaBH4?
The atom holding on to the hydrides should be examined here. In the case of NaBH4 we have boron with an electronegativity of around 2.0 where as with LiAlH4 we have aluminum with an electronegativity around 1.6. Remember, the more electronegative an atom is, the less likely it will be to share or give up electrons. A hydride (H-) is just that, extra electrons (one to be exact) on a hydrogen. So if the hydride is bound to boron, it is less likely to be released for organic transformation when compared to aluminum (because boron will hold on to electrons, and thus the hydride, better).
How to react alkene with H2?
Just react the alkene with H2 in presence of ni/pt/pd (catalyst). You'll get alkane.
When is LAH used?
in point of fact, LAH is used as against olefins (alkenes). Usually when there is a good reason to, say, to reduce multiple functional groups in addition to the alkene.
Which is more stable, alkyl or vinyl?
So the transition state of alkyl cation will be more stable than that of Vinyl cation.
Can a weaker reducing agent reduce alkene?
Otherwise a weaker reducing agent can reduce the olefin (alkene) without requiring harsh reaction conditions. (LAH reacts with common solvents much more than some of the gentler choices.). Hence: overkill.
What reacts with LiAlH4- or AlH3 to produce alkoxide?
The aldehyde then reacts with either LiAlH4- or AlH3 to yield an alkoxide that is then protonated with water or hydronium.
What reacts with AlH3?
In the mechanism, RCO2- Li+ reacts with AlH3....#N#AlH3 donates a hydrogen and coordinates with the oxygen not coordinated to the lithium ion,#N#AlH2O- leaves and an aldehyde intermediate results.#N#The aldehyde intermediate reacts with either AlH3 or AlH4- either of which is a sufficient reducing agent. Then the alkoxide coordinated to the Lithium ion is protonated to yield a primary alcohol.
What is the reaction of ester/carboxylic acid?
Then the reaction is simply a matter of ester/carboxylic acid reduction whereby one oxygen from each terminal functional group is displaced by hydrogen.
Does hydride attack carbonyl carbon?
To that question I would say it does nothing. After hydride attack on the carbonyl carbon, I don't see any possible resonance structures involving movement of the beta unsaturated bond short of regenerating hydride shifts, and that shouldn't happen. It's one bond too far from offering any resonance stabilization.
Does LAH reduce ester carbonyl?
I believe the point everyone is trying to make is that the "answer" shows reduction by LAH to not only reduce the ester carbonyl, but the carboxylic acid to a gem-diol (C4 having 2 -OH groups attached to it), which I am also of the opinion does not happen. LAH is a strong reducing agent and should reduce the carboxy group to a primary alcohol, not a gem-diol, first by reducing the carboxyl to an aldehyde intermediate, and then again to a primary alcohol. Unless the question implies some sort of very mild condition, i'd call this reaction bunk until someone gives a solid reason for the reduction stopping at the gem-diol step
Is carboxylic acid a primary alcohol?
But a carboxylic acids and esters are reduced to PRIMARY alcohols.
Does lithium hydride reduce alcohol?
Lithium aluminium hydride will reduce esters and acids to a primary alcohol.
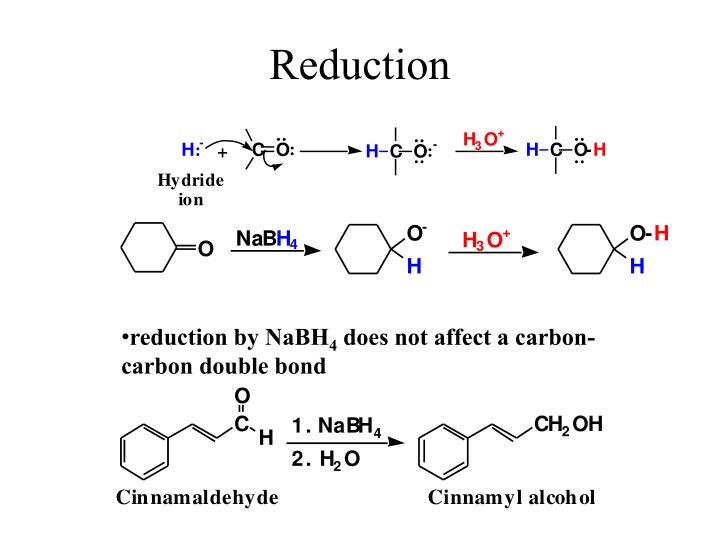
LiAlH4 Reduction of Aldehydes and Ketones – The Mechanism
NaBH4 Reduction of Aldehydes and Ketones – The Mechanism
- Sodium borohydride reduces aldehydes and ketones by a similar mechanism with some important differences that we need to mention. First, NaBH4 is not so reactive and the reaction is usually carried out in protic solvents such as ethanol or methanol. The solvent has two functions here: 1) It serves as the source of a proton (H+) once the reduction is complete 2) The sodium ion is a w…
The Mechanism of LiAlH4 Reduction of Carboxylic Acids
- The reduction of carboxylic acids also requires an excess of LiAlH4. The first reaction between a carboxylic acid and LiAlH4 is simply a Brønsted–Lowry acid-base reaction: The resulting carboxylate is almost unreactive because of the high electron density and this is why reduction of carboxylic acids is more difficult and requires more forcing conditions. One good alternative to t…
The Stereochemistry of LiAlH4 and NaBH4 Reduction
- The reduction of unsymmetrical ketones with LiAlH4 or NaBH4 produces a pair of stereoisomers because the hydride ion can attack either face of the planar carbonyl group: If no other chiral center are present, the product is a racemic mixture of enantiomers.
Alcohols from Catalytic Hydrogenation
- Another common method for preparing alcohols from aldehydes and ketones is the catalytic hydrogenation: Remember, catalytic hydrogenation was the method for reducing alkynes to alkenes or alkanes depending on the specific reagent. And this is the reason why hydride reductions using LiAlH4 and NaBH4 are preferredhen multiple functional groups are present in t…
A Summary For Alcohol Reducing Reagents
- In addition to LiAlH4 and NaBH4, there are hundreds of different hydrides reducing agents designed for specific scenarios and combination of functional groups in the molecule. They all have their advantages and disadvantages. It is impossible to address this in a single article and most of them are beyond the scope of most undergraduate programs. However, below is a sum…