Prepare Potassium Chlorate
- Boil a large volume (at least a half liter) of chlorine bleach, just until crystals start to form. Do this outdoors or...
- As soon as crystals start to form, remove the bleach from heat and allow it to cool.
- In a separate container, prepare a saturated solution of potassium chloride by stirring potassium chloride into the...
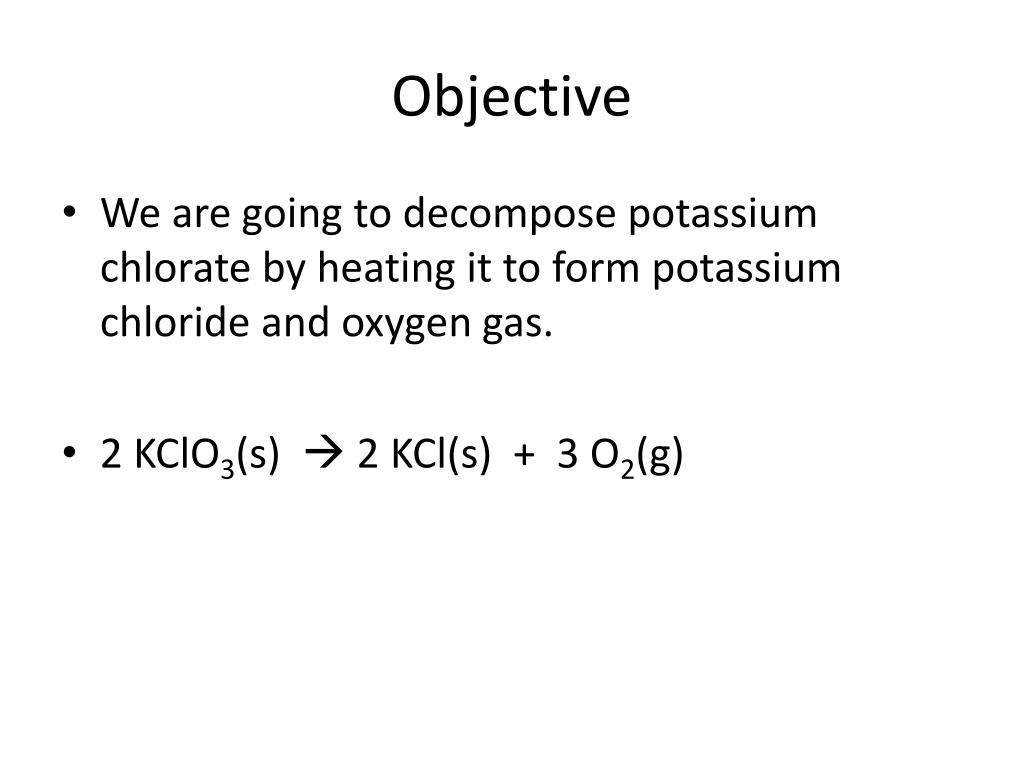
What does potassium chlorate produce if it decomposes?
The general pattern of a decomposition of a chlorate reaction is: MClO3 --> MCl + O2 (M is used to represent the metal. ClO3 is chlorate.) Look at the example below. Example #1: 2KClO3 (s) --> 2KCl (s) + 3O 2 (g) Potassium chlorate decomposes to form potassium chloride and oxygen gas.
What if potassium chlorate is contaminated with KCL?
a) If the potassium chlorate had been contaminated with potassium chloride, the % oxygen would go down. The lack of weight lost would indicate that less mass burned off of the substance.
How does potassium chloride increase heart rate?
Potassium levels above 6,0 mmol/l cause peaked T waves, wider QRS komplexes and may result in bradycardia, asystole and sudden death. How does potassium chloride affect heart rate? Injection of KCl regularly induces acceleration of the heart rate and an increase in blood pressure.
Is potassium chlorate a base or acid?
Potassium chlorate is an ionic compound that is dissociated into K+ and ClO3- ions. So potassium chlorate is neither an acid nor a base. It is a salt formed from the reaction of the acid HClO3 and the base KOH. This is answered comprehensively here. Beside this, is KClO3 ionic or molecular?
How do you make KClO3?
While it's not the most efficient chemical reaction, it's simple to make potassium chlorate by boiling bleach, cooling it, and mixing in a saturated solution of salt substitute in water. The synthesis works because potassium from the salt substitute displaces sodium from the sodium chlorate made by boiling the bleach.
Can potassium chlorate turn into potassium chloride?
2:196:19Potassium Chlorate from Bleach - YouTubeYouTubeStart of suggested clipEnd of suggested clipAnd that is a solution of like I said studying chloride and sodium chlorate. So now we need toMoreAnd that is a solution of like I said studying chloride and sodium chlorate. So now we need to change that to potassium chlorate. So that we can recover it and we're gonna add potassium chloride to do
Is potassium chloride and potassium chlorate the same thing?
Potassium chloride electrolysis also yields potassium chlorate. Electrolysis of KCl in water forms chlorine at the anode, which reacts with potassium hydroxide (KOH) in the liquid. Potassium chlorate formed by the reaction precipitates out of solution.
How potassium chlorate is produced?
Electrolysis Method – Potassium Chlorate can also be produced by electrolysis of Potassium Chloride. In this method, we take anode of carbon, platinum or mixed metal oxide and cathode of titanium. Both the electrodes are inserted in the aqueous solution of Potassium Chloride and a current is passed through.
How do you make potassium chloride naturally?
Protein-Rich Foods Nuts and nut spreads, protein products, canned or fermented seafood, sausage casings, processed meat or poultry and soybeans can all contain potassium chloride. The breading on some meat and poultry products may also be a source of this additive.
What is flash powder made from?
Aluminium and perchlorate Aluminium powder and potassium perchlorate are the only two components of the pyrotechnic industry standard flash powder. It provides a great balance of stability and power, and is the composition used in most commercial exploding fireworks.
What happens when you put a gummy bear in potassium chlorate?
When heated, potassium chlorate decomposes, producing sufficient oxygen to ignite the sugar in the gummy bear. Since the oxidation of the sugar is very exothermic, sodium chlorate continues to decompose to oxygen, and the rate of combustion becomes very rapid.
How do you make salt bleach?
2:186:36Making Bleach (Sodium Hypochlorite) from Table Salt (Sodium Chloride)YouTubeStart of suggested clipEnd of suggested clipAnd the sodium hydroxide in distribution. So the two will react to make sodium hypochlorite orMoreAnd the sodium hydroxide in distribution. So the two will react to make sodium hypochlorite or bleach and other products like sodium chloride.
Is potassium chlorate used in explosives?
Potassium Chlorate is a transparent, colorless crystal or white powder. It is used as an oxidizing agent, and in explosives, matches, textile printing, disinfectants and bleaches.
How do you break down potassium chlorate?
When KClO3 is heated strongly, it breaks down, releasing oxygen gas and leaving behind a thermally stable (i.e., heat-insensitive) solid residue of an ionic potassium compound. There are at least three plausible reactions one can write for the process, but only one occurs to any significant extent.
How do you make potassium chlorate from electrolysis?
2:2019:54Make Potassium Chlorate by Electrolysis - The Basic GuideYouTubeStart of suggested clipEnd of suggested clipNow we turn the current and wait. It's making potassium chlorate but you can see carbon particlesMoreNow we turn the current and wait. It's making potassium chlorate but you can see carbon particles slough off the carbon electrode and contaminate the solution.
Why potassium chlorate is banned for use in fireworks?
It has an inherent property to become very reactive, especially when mixed with sulphur; the potassium chlorate-sulphur mixture becomes dangerously sensitive to friction and may spontaneously ignite. Hence, potassium chlorate is banned for use in fireworks.
How to make potassium chlorate?
Potassium chlorate is a useful oxidizer and small amounts can be easily made using household chemicals. Start by boiling a large quantity of household laundry bleach, at least half a liter, until crystals start to precipitate. Immediately take it off heating and let it cool.
Can you mix potassium chloride with bleach?
Potassium chloride is sold as a "sodium-free" salt substitute. Now, once the bleach is cooled, measure out an equal volume of potassium chloride solution and pour into the boiled bleach solution, but do not mix in the crystals. Stir up the mixture and eventually potassium chlorate crystals will precipitate out.
What is the first target for making chlorate?
2OH - + Cl 2 -> 2 ClO - + H 2. thus generating hypochlorite. The first target for making chlorate however is hypochlorite, to be oxidized to chlorate, so this process must generate hypochlorite and therefore mixing should take place.
What is KClO3?
Potassium chlorate KClO 3 (also called Potassii Chloras or Kalii Chloras or Chloras Kalicus/Potassicus) is the potassium salt of chloric acid and is an oxidizer, used in chemistry to produce oxygen, but also used in fireworks. It is obtainable at chemicals supply shops, such as Merck or other large suppliers.
How much KCl is in diet salt?
Pour this into the jar (carefully, as it will break otherwise at the quick temperature change). Put the thermometer in the jar. Newer diet salt consists of even 67% KCl and 33% NaCl, which is even better. Connect the anode with the red (+) clamp of the supply and the cathode with the black (or white) (-) clamp.
How to get crystals out of a crystal beaker?
Pour some ice-cold water (from the fridge or freezer) over the crystals (no more volume than the crystals) to rinse off other salt solution. Pour off any water and put the beaker with the crystals on a heat source and heat is with a very soft flame. Allow any water to evaporate.
Is potassium chlorate a strong oxidizer?
Potassium chlorate (KClO3) is a strong oxidizer. Do not heat or rub it with combustibles, like carbon, sugar, etc. With Phosphorus or even sulphur in may ignite easily. Avoid contact with acids as well.
Can KClO3 be dissolved in cold water?
The raw crystals after cooling down the filtrate. A winter way for cooling the solution. As KClO 3 has a poor solubility in cold water, but a good one in hot water, while other salts (like NaCl, KCl and NaClO 3) dissolve much better in cold water, one can let crystallize out the KClO 3 and obtain it rather purely.
How are chlorates prepared?
On an industrial scale, chlorates are prepared by electrolysis. Electrolysing a solution of a chloride at elevated temperatures yields a chlorate. This method can be down scaled quite easily for amateur pyro purposes. Other methods of chlorate manufacture exist that may be of interest for small scale use.
What is the mechanism of chlorate formation?
Mechanism of chlorate formation. The reactions taking place in chlorate cells are not fully understood even today. The theory of Foerster and Mueller regarding the reactions in chlorate cells, developed about 80 years ago, is the most accepted. The following reactions are said to take place at the electrodes:
How is chlorate formed?
Alternatively, chlorate may also be formed by oxidation of hypochlorite at the anode as follows: 6HClO + 3 H 2 O 3 / 2 O 2 + 4Cl - + 2ClO 3- + 12H + + 6 electrons.
Why is chlorate bad?
One of the main problems in chlorate cells is the corrosiveness of the electrolyte. Only very few materials do not corrode when in contact with the electrolyte or its fumes. Most metals corrode, many plastics will and even glass does under some circumstances.
How many faradays of charge are needed to produce 1 mole of chlorate?
When the reaction routes are worked out, it turns out that following this path 9 faradays of charge are required to produce 1 mole of chlorate, whereas only 6 faradays are required to do that following the route mentioned earlier. Therefore, optimising the conditions for that route improves current efficiency.
What is electrolysis preparation?
Electrolytic preparation. The electrolysis is carried out in a diaphragm less cell, containing a solution of a chloride. Several chlorides may be used, but the use of sodium chloride has many advantages. Sodium chlorate is easily converted to a number of other chlorates by metathesis reactions.
What is the reaction of hypochlorous acid?
The hypochlorous acid thus formed will react in acid-base equilibrium reactions with water to give hypochlorite ions and chlorine gas (dissolved). The exact concentrations of dissolved Cl 2, ClO - and HClO depend on the pH, temperature and pressure among other things.