Number of Neutrons = Mass Number - Number of Protons = 1 - 1 = 0 For zinc, the atomic
Nuclear weapon
A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission (fission bomb) or a combination of fission and fusion (thermonuclear weapon). Both reactions release vast quantities of energy from relatively small amounts of matter.
How do you find the number of protons in zinc zinc?
The nucleus is made up of protons and neutrons. Find the element Zn Zn on the periodic table. To find the number of protons in zinc zinc, first locate the element on the periodic table.
How do you find the number of neutrons in an atom?
the atomic number) from the atomic mass will give you the calculated number of neutrons in the atom. The numbers after the decimal point represent the usually very small mass of the electrons in the atom.
What is the mass number and neutron number of Zn?
Zinc - Mass Number - Neutron Number - Zn. Mass numbers of typical isotopes of Zinc are 64; 66-68; 70. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N.
How many valence electrons are in a zinc atom?
This means that neutral zinc atom has a total of 30 electrons surrounding its nucleus. To see how many of these electrons are valence electrons, write the electron configuration of a neutral zinc atom. Herein, how do you find the number of neutrons?
See more
What is the correct way to find the number of neutrons?
To find the number of neutrons, subtract the number of protons from the mass number. number of neutrons=40−19=21.Feb 6, 2021
How many neutron are there in Zn?
35 neutronsZinc-65 atom is a zinc atom in which the nucleus contains 35 neutrons....4.3Related Element.Element NameZincElement SymbolZnAtomic Number30
How many neutrons does zinc 66?
36Properties of Zinc-66 Isotope:Properties of Zinc-66 Isotope:ZINC-66Neutron Number (N)36Atomic Number (Z)30Mass Number (A)66Nucleon Number (A)6625 more rows
How many protons and neutrons are in zinc?
30Zinc / Atomic number
How to find the atomic mass of zinc?
Average Atomic Mass. To find the number of protons in zinc zinc, first locate the element on the periodic table. Next, find the atomic number which is located above the element 's symbol. Since zinc zinc 's atomic number is 30 30, Zn Zn has 30 30 protons. 30 30 protons. Fill in the known values where N N represents the number of neutrons.
How to find the atomic weight of an atom?
So the first step is to locate the atomic mass on the periodic table , and round the value to the nearest whole number. 65.39 65.39 round to 65 65.
What is the nucleus made of?
The nucleus is made up of protons and neutrons. Atomic Mass = Number of Protons+Number of Neutrons Atomic Mass = Number of Protons + Number of Neutrons. Find the element Zn Zn on the periodic table. 30. Atomic Number.
How to find the number of neutrons in an atom?
Categories: Chemistry. Article Summary X. To find the number of neutrons in an atom, start by locating the element on the periodic table. Then, find the element's atom number, which is usually in the top left or middle of the box above the element symbol.
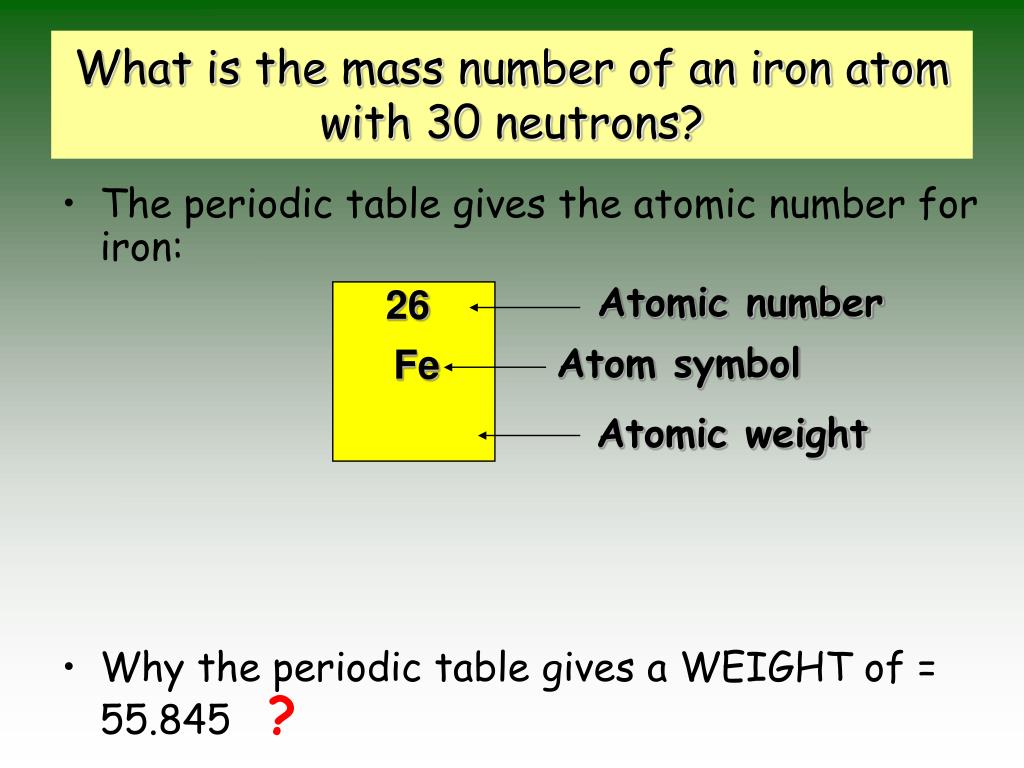
Where is the atomic number on a chart?
This tends to be the most visible number pertaining to a given element and usually sits above the element symbol, either in the middle of the box or in the upper left corner. (On the chart we're using, in fact, no other numbers are listed.) The atomic number is the number of protons in a single atom of that element.
What is the atomic number of an element?
The atomic number is the number of protons in a single atom of that element. Os is number 76, meaning one atom of osmium has 76 protons. The proton number never changes in an element; it's basically what makes that element that element.
How to tell if an atom is an isotope?
Although all atoms of the same element contain the same number of protons, their number of neutrons can vary. Knowing how many neutrons are in a particular atom can help you determine if it's a regular atom of that element or an isotope, which will have either extra or fewer neutrons. Determining the number of neutrons in an atom is fairly simple ...
What is the atomic number of a periodic table?
If you’re ever unsure which number is which in a periodic table, just remember that the table is usually designed around the atomic number (i.e. number of protons), which starts at 1 (hydrogen) and ascends one unit at a time from left to right, ending at 118 (oganesson).
What is the empty space between the atomic cloud of an atom and its nucleus?
The empty space between the atomic cloud of an atom and its nucleus is just that: empty space, or vacuum. That's the simple answer, but there are a few subtleties: Subatomic particles such as electrons, protons and neutrons need to be treated as quantum objects.
How to find neutrons?
Subtract the atomic number from the atomic mass. To find the number of neutrons, you will need to subtract the atomic number from the atomic mass. Remember that the atomic number is the same as the number of protons, which you have already identified.
How to find the number of protons, neutrons, and electrons?
The easiest way to find the number of protons, neutrons, and electrons for an element is to look at the element’s atomic number on the periodic table. That number is equal to the number of protons. The number of protons is equal to the number of electrons, unless there’s an ion superscript listed after the element.
How to find the amount of protons and electrons in an atom?
You need the atomic number to find the amount of protons and/or electrons, unless you have the amount of neutrons and the atomic mass, in which case you can simply subtract the amount of neutrons from the atomic mass, leaving the amount of protons in the atom.
How many protons are in actinium?
Expert Answer. You will find actinium in group 3, period 7 of the periodic table. The atomic number of actinium is 89, which means there are 89 protons. Because there is no net charge, we know that # protons = # electrons, so there are 89 electrons as well.
How to find the remaining number of electrons in an ion?
To calculate the remaining number of electrons, you subtract the amount of extra charge from the atomic number. In the case of a positive ion, there are more protons than electrons. For example, Ca 2+ has a +2 charge so it has lost 2 electrons from the neutral state.
How to find the atomic number of an element?
Locate the element’s atomic number. The atomic number is located above the element symbol, in the upper left-hand corner of the square. The atomic number will tell you how many protons make up a single atom of an element. For example, boron (B) has an atomic number of 5, therefore it has 5 protons.
How many electrons does calcium have?
Calcium’s atomic number is 20, therefore the ion has 18 electrons. Add the charge to the atomic number for negative ions. When an ion has a negative charge, the atom has gained electrons. To calculate the total number of present electrons, you simply add the amount of extra charge to the atomic number.