How is the atomic weight of beryllium determined?
Beryllium is a monoisotopic element and its atomic weight is determined solely by its isotope 9 Be. The Commission last revised the standard atomic weight of beryllium in 2013 based on the latest Atomic Mass Evaluation by IUPAP. Before 1961, the atomic weight of beryllium was based on chemical determinations.
How do you extract beryllium from Beryl?
Extraction of beryllium using the melt method involves grinding beryl into a powder and heating it to 1,650 °C (3,000 °F). The melt is quickly cooled with water and then reheated 250 to 300 °C (482 to 572 °F) in concentrated sulfuric acid, mostly yielding beryllium sulfate and aluminium sulfate.
What is the concentration of beryllium in the atmosphere?
Beryllium has a concentration of 2 to 6 parts per million (ppm) in the Earth's crust. It is most concentrated in the soils, 6 ppm. Trace amounts of 9 Be are found in the Earth's atmosphere. The concentration of beryllium in sea water is 0.2–0.6 parts per trillion.
How many protons and electrons are in beryllium?
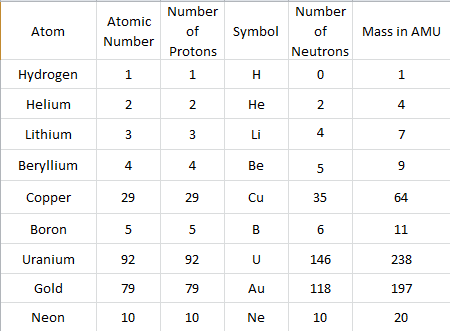
Beryllium is a chemical element with atomic number 4 which means there are 4 protons and 4 electrons in the atomic structure. The chemical symbol for Beryllium is Be. Beryllium is a hard, grayish metal naturally found in mineral rocks, coal, soil, and volcanic dust.
See more
What is the atomic mass for beryllium?
9.012182 uBeryllium / Atomic mass
How do we find atomic mass?
Together, the number of protons and the number of neutrons determine an element's mass number: mass number = protons + neutrons. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from the mass number.
What is the easiest way to find the atomic mass?
To calculate the atomic mass of a single atom of an element, add up the mass of protons and neutrons. Example: Find the atomic mass of an isotope of carbon that has 7 neutrons. You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons.
How do you find the atomic number?
The atomic number of an atom is equal to the number of protons in the nucleus of an atom or the number of electrons in an electrically neutral atom. For example, in a sodium atom, there are 11 electrons and 11 protons. Thus the atomic number of Na atom = number of electrons = number of protons = 11.
How do you find the atomic mass of protons neutrons and electrons?
4:0113:12How To Calculate The Number of Protons, Neutrons, and ElectronsYouTubeStart of suggested clipEnd of suggested clipSo for ions the number of electrons and protons are different but for atoms which are electricallyMoreSo for ions the number of electrons and protons are different but for atoms which are electrically neutral the number of protons and electrons are the same. So let's start with this example go ahead
How do you calculate atomic mass Wikihow?
Add the proton and neutron count. Our carbon atom has 6 protons + 6 neutrons = 12. The atomic mass of this specific carbon atom is 12. If it was a carbon-13 isotope, on the other hand, we would know that it has 6 protons + 7 neutrons = an atomic weight of 13.
How do you learn the atomic mass of the first 20 elements trick?
2:535:18Trick to Learn First 20 Elements of the Periodic Table - YouTubeYouTubeStart of suggested clipEnd of suggested clipRemember that we use two common trigs for instance trick for the even atomic numbers and trick forMoreRemember that we use two common trigs for instance trick for the even atomic numbers and trick for the odd atomic. Numbers in case of even atomic. Numbers double it while in case of odd atomic.
How do you find the atomic mass of the first 30 elements?
The atomic mass of elements is measured with the help of unified atomic mass units. One unified atomic mass unit can be quantified as the weight of one-twelfth of the mass of a carbon-12 atom considering that it is at rest....Atomic Mass of First 30 Elements.ATOMIC NUMBERELEMENTATOMIC MASS28Nickel58.69329Copper63.54630Zinc65.3827 more rows
Is atomic mass the atomic number?
Atomic mass is associated with the number of neutrons and protons that are present in a particular nucleus of an element. Atomic number is usually the number of protons present in an element's nucleus. It is the average weight of an element.
Is atomic mass and mass number same?
Key Takeaways: Atomic Mass Versus Mass Number The mass number is the sum of the number of protons and neutrons in an atom. It is a whole number. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. It is a decimal number.
What makes up the atomic mass?
The atomic mass of an atom is an empirically measured property, which is equivalent to the sum mass of protons, neutrons, and electrons that make up the atom (with a small adjustment for nuclear binding energy).
What is the atomic mass of beryllium?
Atomic Mass of Beryllium. Atomic mass of Beryllium is 9.0122 u . Note that, each element may contain more isotopes, therefore this resulting atomic mass is calculated from naturally-occuring isotopes and their abundance. The unit of measure for mass is the atomic mass unit (amu).
How many grams are in one atomic mass unit?
One atomic mass unit is equal to 1.66 x 10-24 grams. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol. For 12C the atomic mass is exactly 12u, since the atomic mass unit is defined from it.
What is the atomic mass of 12C?
For 12C the atomic mass is exactly 12u , since the atomic mass unit is defined from it. For other isotopes, the isotopic mass usually differs and is usually within 0.1 u of the mass number. For example, 63Cu (29 protons and 34 neutrons) has a mass number of 63 and an isotopic mass in its nuclear ground state is 62.91367 u.
What is density in math?
Density is defined as the mass per unit volume. It is an intensive property, which is mathematically defined as mass divided by volume: ρ = m/V. In words, the density (ρ) of a substance is the total mass (m) of that substance divided by the total volume (V) occupied by that substance.
Which is heavier, a proton or a neutron?
The neutron is slightly heavier than the proton. This increases the mass of nuclei with more neutrons than protons relative to the atomic mass unit scale based on 12C with equal numbers of protons and neutrons. The nuclear binding energy varies between nuclei.
What is the oxidation state of beryllium?
A beryllium atom has the electronic configuration [He] 2s 2. The predominant oxidation state of beryllium is +2; the beryllium atom has lost both of its valence electrons. Lower oxidation states have been found in, for example, bis (carbene) compounds.
What is the symbol for beryllium?
Category: Beryllium. view. talk. edit. | references. Beryllium is a chemical element with the symbol Be and atomic number 4. It is a steel-gray, strong, lightweight and brittle alkaline earth metal. It is a divalent element that occurs naturally only in combination with other elements to form minerals.
What is the shortest-lived isotope of beryllium?
The shortest-lived known isotope of beryllium is 13 Be which decays through neutron emission. It has a half-life of 2.7 × 10 −21 s. 6 Be is also very short-lived with a half-life of 5.0 × 10 −21 s. The exotic isotopes 11 Be and 14 Be are known to exhibit a nuclear halo.
How to extract beryllium from a sulfate?
Extraction of beryllium using the melt method involves grinding beryl into a powder and heating it to 1,650 °C (3,000 °F). The melt is quickly cooled with water and then reheated 250 to 300 °C (482 to 572 °F) in concentrated sulfuric acid, mostly yielding beryllium sulfate and aluminium sulfate.
What is the neutron multiplier in a nuclear reaction?
Thus, for high-energy neutrons, beryllium is a neutron multiplier, releasing more neutrons than it absorbs. This nuclear reaction is: Neutrons are liberated when beryllium nuclei are struck by energetic alpha particles producing the nuclear reaction. is a carbon-12 nucleus.
How is beryl extracted?
Beryllium is most commonly extracted from the mineral beryl, which is either sintered using an extraction agent or melted into a soluble mixture. The sintering process involves mixing beryl with sodium fluorosilicate and soda at 770 °C (1,420 °F) to form sodium fluoroberyllate, aluminium oxide and silicon dioxide.
How much beryllium is in sea water?
The concentration of beryllium in sea water is 0.2–0.6 parts per trillion. In stream water, however, beryllium is more abundant with a concentration of 0.1 ppb. Beryllium is found in over 100 minerals, but most are uncommon to rare.
How are atomic nuclei determined?
Properties of atomic nuclei (atomic mass, nuclear cross-sections) are determined by the number of protons and number of neutrons (neutron number). It must be noted, especially nuclear cross-sections may vary by many orders from nuclide with the neutron number N to nuclide with the neutron number N+1. For example, actinides with odd neutron number are usually fissile (fissionable with slow neutrons) while actinides with even neutron number are usually not fissile (but are fissionable with fast neutrons). Heavy nuclei with an even number of protons and an even number of neutrons are (due to Pauli exclusion principle) very stable thanks to the occurrence of ‘paired spin’. On the other hand, nuclei with an odd number of protons and neutrons are mostly unstable.
How to determine the stability of an isotope?
To determine the stability of an isotope you can use the ratio neutron/proton (N/Z). Also to help understand this concept there is a chart of the nuclides, known as a Segre chart. This chart shows a plot of the known nuclides as a function of their atomic and neutron numbers. It can be observed from the chart that there are more neutrons than protons in nuclides with Z greater than about 20 (Calcium). These extra neutrons are necessary for stability of the heavier nuclei. The excess neutrons act somewhat like nuclear glue. Only two stable nuclides have fewer neutrons than protons: hydrogen-1 and helium-3.
When was the atomic weight of beryllium revised?
The Commission last revised the standard atomic weight of beryllium in 2013 based on the latest Atomic Mass Evaluation by IUPAP. Before 1961, the atomic weight of beryllium was based on chemical determinations.
Who discovered beryllium?
Beryllium was discovered by the French chemist and pharmacist Nicholas-Louis Vauquelin in beryl and emerald in 1797. The element was first separated in 1828 by the French chemist Antoine-Alexandre-Brutus Bussy and independently by the German chemist Friedrich Wöhler. Because the salts of beryllium have a sweet taste, ...
Why is beryllium called glucinium?
Because the salts of beryllium have a sweet taste, the element was also known as glucinium from the Greek glykys for "sweet", until IUPAC selected the name beryllium in 1949.
Is beryllium a monoisotopic element?
Beryllium is the only monoisotopic element with an even atomic number, but it has an odd mass number like all other monoisotopic elements. The name derives from the Greek word beryllos for "beryl", a gemstone in which it is found (3BeO×Al 2 O 3 ×6SiO 2 ). Beryllium was discovered by the French chemist and pharmacist Nicholas-Louis Vauquelin in ...
Computing molar mass (molar weight)
To calculate molar mass of a chemical compound enter its formula and click 'Compute'. In chemical formula you may use:
Computing molecular weight (molecular mass)
To calculate molecular weight of a chemical compound enter it's formula, specify its isotope mass number after each element in square brackets.
Definitions of molecular mass, molecular weight, molar mass and molar weight
Molecular mass ( molecular weight) is the mass of one molecule of a substance and is expressed in the unified atomic mass units (u). (1 u is equal to 1/12 the mass of one atom of carbon-12)
What is the electron configuration of beryllium?
Electron configuration of Beryllium is [He] 2s2. Possible oxidation states are +1; +2. Beryllium reacts with acids and with water to form hydrogen gas. It reacts briefly with oxygen in the air to form beryllium oxide (BeO).
How many protons are in a beryllium atom?
Beryllium is a chemical element with atomic number 4 which means there are 4 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.
How many electrons are in a neutral atom of beryllium?
Therefore, the number of electrons in neutral atom of Beryllium is 4. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative electrons in the atom.
What is a beryllium?
Beryllium is a hard, grayish metal naturally found in mineral rocks, coal, soil, and volcanic dust. Beryllium has a large scattering cross section for high-energy neutrons, about 6 barns for energies above approximately 10 keV. Therefore, it works as a neutron reflector and neutron moderator, effectively slowing the neutrons to the thermal energy.
What is the total electrical charge of the nucleus?
The total electrical charge of the nucleus is therefore +Ze , where e (elementary charge) equals to 1,602 x 10-19 coulombs. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. Neutron number plus atomic number equals atomic mass number: N+Z=A.
What determines the chemical bonding behavior of an element?
The configuration of these electrons follows from the principles of quantum mechanics. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior.
Which element has an odd number of neutrons?
Beryllium is unique as being the only monoisotopic element with both an even number of protons and an odd number of neutrons.
Overview
History
The mineral beryl, which contains beryllium, has been used at least since the Ptolemaic dynasty of Egypt. In the first century CE, Roman naturalist Pliny the Elder mentioned in his encyclopedia Natural History that beryl and emerald ("smaragdus") were similar. The Papyrus Graecus Holmiensis, written in the third or fourth century CE, contains notes on how to prepare artificial emerald and b…
Characteristics
Beryllium is a steel gray and hard metal that is brittle at room temperature and has a close-packed hexagonal crystal structure. It has exceptional stiffness (Young's modulus 287 GPa) and a melting point of 1287 °C. The modulus of elasticity of beryllium is approximately 50% greater than that of steel. The combination of this modulus and a relatively low density results in an unusuall…
Production
The extraction of beryllium from its compounds is a difficult process due to its high affinity for oxygen at elevated temperatures, and its ability to reduce water when its oxide film is removed. Currently the United States, China and Kazakhstan are the only three countries involved in the industrial-scale extraction of beryllium. Kazakhstan produces beryllium from a concentrate stockpiled before the breakup of the Soviet Union around 1991. This resource has become nearl…
Chemical properties
A beryllium atom has the electronic configuration [He] 2s . The predominant oxidation state of beryllium is +2; the beryllium atom has lost both of its valence electrons. Lower oxidation states have been found in, for example, bis(carbene) compounds. Beryllium's chemical behavior is largely a result of its small atomic and ionic radii. It thus has very high ionization potentials and strong polarization …
Applications
Because of its low atomic number and very low absorption for X-rays, the oldest and still one of the most important applications of beryllium is in radiation windows for X-ray tubes. Extreme demands are placed on purity and cleanliness of beryllium to avoid artifacts in the X-ray images. Thin beryllium foils are used as radiation windows for X-ray detectors, and the extremely low absorption mi…
Occupational safety and health
Beryllium is a health and safety issue for workers. Exposure to beryllium in the workplace can lead to a sensitization immune response and can over time develop chronic beryllium disease (CBD). The National Institute for Occupational Safety and Health (NIOSH) in the United States researches these effects in collaboration with a major manufacturer of beryllium products. The goal of this research is to prevent sensitization and CBD by developing a better understanding of the work pr…
Precautions
Approximately 35 micrograms of beryllium is found in the average human body, an amount not considered harmful. Beryllium is chemically similar to magnesium and therefore can displace it from enzymes, which causes them to malfunction. Because Be is a highly charged and small ion, it can easily get into many tissues and cells, where it specifically targets cell nuclei, inhibiting many enzymes, including those used for synthesizing DNA. Its toxicity is exacerbated by the fact that t…