Calculate H3O+ when you know OH-: (H3O+) = (1 * 10-14) / (OH-) Calculate OH- when you know H3O+: (OH-) = (1 * 10-14) / (H3O+) Calculate H3O+ from OH- Example 1: The hydroxide ion concentration is known to be 4. Video advice: pH, [H3O+], & [OH-] Calculations Watch this video on YouTube
What is the solution for H3O OH?
In neutral aqueous solutions at 25 °C, the [H30+] = [OH-] . If the solution is acidic such as vinegar (acetic acid in water), the [H3O+] > [OH-]. If the solution is basic such as lye (NaOH) in water, then the reverse is true.
What are the concentrations of H3O and Oh?
The relative concentrations of hydronium and hydroxide ion in a solution define its status as acidic ( [H3O+] > [OH−]), basic ( [H3O+] < [OH−]), or neutral ( [H3O+] = [OH−]). At 25 °C, a pH < 7 indicates an acidic solution, a pH > 7 a basic solution, and a pH = 7 a neutral solution. What is the pH of CsOH? Is CsOH acid or base?
How do you find the pH of Oh-?
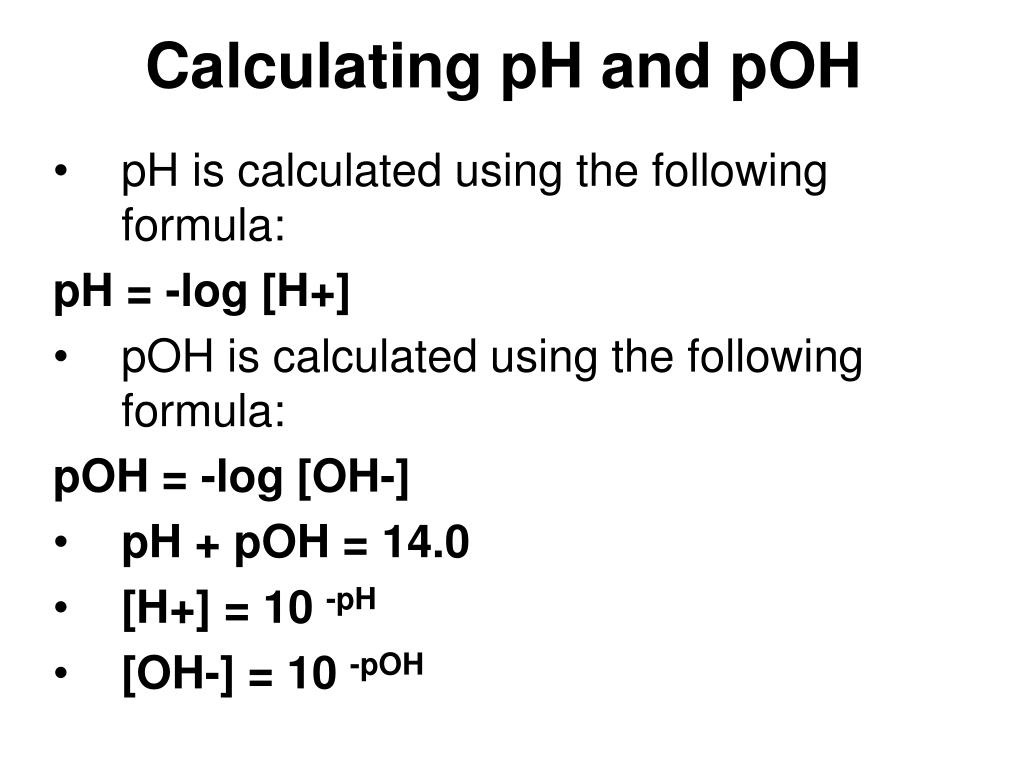
pH=-log[H+] 2. pOH=-log [OH-] 3. pH + pOH=14 4. [H+]=2nd log (-pH) 5. [OH-]= 2nd log (-pOH) 6. [H+] x [OH-]=1X10-14 **Log is a function on the calculator and stands for logarithm!
How many moles of OH produced from hydroxides?
one mole of barium hydroxide contain one mole of Ba²⁺ and 2 mole of OH⁻ ion. where are the choice options? Aside from human impact, which of the following causes less water vapor production over a small area? (2 pderivartin
How do you find h3o when given OH?
0:242:35Calculate H₃O⁺ from OH⁻ - YouTubeYouTubeStart of suggested clipEnd of suggested clipOkay kW itself equals one point O. So we've got to isolate the variable h3o plus and so what we doMoreOkay kW itself equals one point O. So we've got to isolate the variable h3o plus and so what we do is it in order to do that we divide both sides by the concentration of away one cancel.
How is h3o+ related to OH?
When there is a reaction in an aqueous solution, the water molecules have the ability to attract and temporarily hold a donated proton (H+). This creates the hydronium ion (H3O+). In an acidic aqueous solution, the concentration of hydronium ions will be higher than the concentration of hydroxide (OH-) ions.
How are h3o+ and OH formed?
H3O+ and OH- are also both related acid base reactions. A base will form OH- while an acid will form H3O+. Both reactions for acids and bases will start out with either a base or acid and H2O. The H3O+ and OH- is formed as a product from the H2O in both cases.
How do you find h3o from pH?
0:192:12Calculate pH from [H3O+] and vice versa - YouTubeYouTubeStart of suggested clipEnd of suggested clipWhat is pH. The important relationship to know here is that pH is equal to the negative log of theMoreWhat is pH. The important relationship to know here is that pH is equal to the negative log of the hydronium ion concentration and this is an easy problem to solve. You just substitute in the
Is H3O the same as OH?
0:302:37H+ vs H3O+ (Hydrogen cation vs Hydronium ion) - YouTubeYouTubeStart of suggested clipEnd of suggested clipThen we lose all of the electrons that's the only one that's there so we end up with just a proton aMoreThen we lose all of the electrons that's the only one that's there so we end up with just a proton a positively charged proton. And that's what this represents. It's a proton then when we lose those
How do you find H3O+ from molarity?
1:343:44Calculating pH and H3O+ Contentration - YouTubeYouTubeStart of suggested clipEnd of suggested clipAnd so in this case you'll use the formula pH is minus log h3o plus or H+ concentration.MoreAnd so in this case you'll use the formula pH is minus log h3o plus or H+ concentration.
How do you find H3O and OH from pH?
2:309:31Calculating pH, pOH, [OH] and [H3O] - Real Chemistry - YouTubeYouTubeStart of suggested clipEnd of suggested clipYou just take the negative log of the hydronium ion concentration anytime. You see that P itMoreYou just take the negative log of the hydronium ion concentration anytime. You see that P it actually means negative log so Poh. Then is negative log of my OAH minus concentration.
How do you make H3O?
0:001:17A step-by-step explanation of how to draw the H3O+ Lewis Structure.YouTubeStart of suggested clipEnd of suggested clipPlus the hydronium ion on the periodic table hydrogen's in group one one valence electron but weMorePlus the hydronium ion on the periodic table hydrogen's in group one one valence electron but we have three hydrogens plus oxygen group six or sixteen six valence electrons.
How do you find pH from OH?
4:579:13Calculating pH from [OH-] hydroxide Concentration - CLEAR & SIMPLEYouTubeStart of suggested clipEnd of suggested clipSo we're going to go our two steps again step one let's use the formula of the hydrogen ionMoreSo we're going to go our two steps again step one let's use the formula of the hydrogen ion concentration. Multiplied by the hydroxide ion concentration equals. One times 10 to the negative 14.
How do you find the H3O of a solution?
The hydronium ion concentration can be found from the pH by the reverse of the mathematical operation employed to find the pH. [H3O+] = 10-pH or [H3O+] = antilog (- pH) Example: What is the hydronium ion concentration in a solution that has a pH of 8.34? On a calculator, calculate 10-8.34, or "inverse" log ( - 8.34).
How do you calculate hydronium ion concentration from hydroxide ion concentration?
1:162:08Hydrogen Ion and Hydroxide Ion Concentrations (Example) - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo this is an acidic solution and then we can calculate the pH. So the pH is log base 10 and then weMoreSo this is an acidic solution and then we can calculate the pH. So the pH is log base 10 and then we're given the h3o plus concentration this also of course is the pH of an acidic solution.
What is the relationship between OH -] and pH?
As a solution gets more basic (higher [OH-]), the pH increases. As the pH of a solution decreases by one pH unit, the concentration of H+ increases by ten times. As the pH of a solution increases by one pH unit, the concentration of OH- increases by ten times.
How do you calculate H3O and OH?
7:009:31Calculating pH, pOH, (OH) and (H3O) - Real Chemistry - YouTubeYouTubeStart of suggested clipEnd of suggested clipWe're gonna get Poh equals...
How do you find H3O and OH from pH?
The hydronium ion concentration can be found from the pH by the reverse of the mathematical operation employed to find the pH. (H3O+) = 10-pH or (H...
How do you calculate the OH of a solution?
0:043:48How to Calculate Hydroxide ion (OH-) Concentration from pH - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo ph is simply give...
What is the formula of H3O?
H₃O⁺Hydronium / Formula.
What is the relationship between H3O +) and OH − for the solutions?
Re: Inverse Relationship Between H3O+ and OH- (ENDORSED) H3O+ and OH- are in equilibrium with water. 2H20<-->H3O+ + OH-. Therefore their Kc(written...
What is the pH of OH?
where a (OH) is the activity coefficient of OH-. Bottom line is the the pH will be close to 14 but maybe a little less.
How much HCl is in a mol?
Stoichiometrically, you can observe that 1 mol of HCl gives 1 mol of H + (aq).Understably, we can infer that 0.1 mol of HCl gives 0.1 mol of H + (aq). If you have an HCl (aq) solution of concentration 0.100 mol dm − 3, then you can say that the concentration of H + (aq) must be 0.100 mol dm − 3 as well.
What does 0.1 mean in molarity?
As molarity of HCl is given 0.1 this means concentration i.e. no. of moles per volume is 0.1 for HCl and same will be for [H+]. As each molecule of HCl gives out 1 H+ ion on ionisation.
What does K a tell us about an acid?
Since they do not fully dissociate, you have consider their K a values as well. K a tells us how much an acid dissociates. Calculations get a bit tricker so I’ll just leave pH calculations of weak acids here.
Is OH ion less or more concentrated?
concentration of OH- ion is so less that it can easily be neglected as compaired
Is NaOH a strong base?
Using these equations, there are a couple of ways you can find your answers. First, you need to know that NaOH is a strong base, this means that the dissociation of NaOH into its ions is 100%. NaOH (s) --> Na+ (aq) + OH- (aq) is 100% forward reaction, therefore [OH-] = [NaOH] = 0.65 M.