The formula to convert Unified Atomic Mass Unit to Joule is 1 Unified Atomic Mass Unit = 1.4924179527347E-10 Joule. Unified Atomic Mass Unit is 6700616456.71402 times Smaller than Joule.
How to convert atoms to Amu?
How do you convert dalton to amu?
- dalton. 0.9999938574 u.
- dalton. 1.9999877148 u.
- dalton.
How do you convert grams to Amu?
Results and discussion
- Free fatty acid analysis. The specific fatty acids were determined through direct polar stationary phases using gas chromatography–mass spectrometry 43.
- Wet lab analysis. ...
- Spectral analysis. ...
- Thermogravimetric analysis. ...
- Surface morphologies. ...
- Microcalorimeter. ...
- Residual char analysis. ...
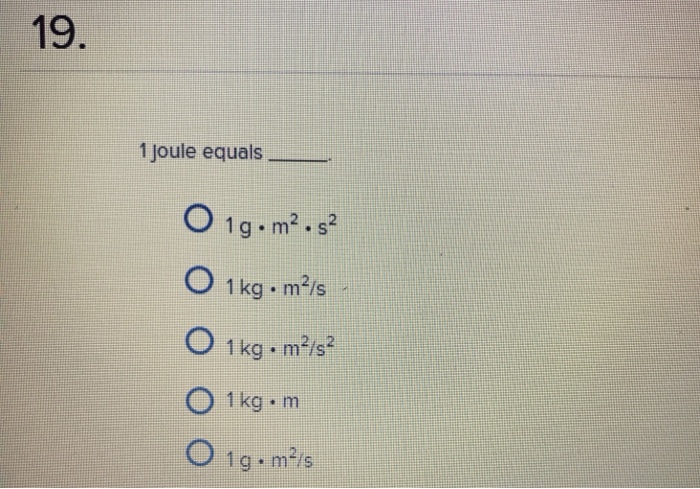
What units is a Joule equivalent to?
What is Joule (unit J) – Energy Unit – Definition
- Energy Units. Energy is generally defined as the potential to do work or produce heat. ...
- Joule – Energy Units. Joule (unit: J). Joule is a derived unit of energy. ...
- Examples of Energy of 1 Joule. The kinetic energy of an object with mass 1 kg moving at √2 ≈ 1.4 m/s. ...
How do you calculate AMU in chemistry?
What About Compounds?
- Write out the chemical formula of the compound. For example: H2O.
- Next, check the periodic table for the atomic mass of the elements used. Example: Hydrogen = 1.0079, Oxygen= 15.9994.
- Next multiply the number of atoms of each element by the atomic mass of the element. ...
- Finally, add the multiplication results. ...
What is the AMU equal to?
In imprecise terms, one AMU is the average of the proton rest mass and the neutron rest mass. This is approximately 1.67377 x 10 -27 kilogram (kg), or 1.67377 x 10 -24 gram (g). The mass of an atom in AMU is roughly equal to the sum of the number of protons and neutrons in the nucleus.
What is the SI unit of 1 AMU?
1 AMU = Average of the proton rest mass and the neutron rest mass. 1 AMU = 1.67377 x 10 -27 kilogram or 1.67377 x 10 -24 gram.
Is AMU and g same?
Gram is used in our day to day life to express the mass of goods that we use whereas amu is used for minute scale measurements. The main difference between amu and grams is that amu is used to express the mass in atomic level whereas gram is used as a metric unit of mass.
Is a proton 1 amu?
A proton is one of three main particles that make up the atom. Protons are found in the nucleus of the atom. This is a tiny, dense region at the center of the atom. Protons have a positive electrical charge of one (+1) and a mass of 1 atomic mass unit (amu), which is about 1.67×10−27 kilograms.
Is an electron 1 amu?
electron: a subatomic particle found outside the nucleus of an atom. It has charge of −1 and a mass of 0 amu (really about 1/2000 amu).
How do you find amu value?
1 amu = Average of the proton rest mass and the neutron rest mass. 1amu = 1.662 x 10-24g 0r 1.662 x 10-27kg. 1amu = 1.662 x 10-24g 0r 1.662 x 10-27kg.
What is the value of 1 amu in gram?
1 amu is equal to 1.66 × 10^-24 g.
How is amu related to grams?
One AMU is equivalent to 1.66 x 10-24 grams. One gram is equivalent to 6.022 x 1023 AMU.
How do you convert amu to energy?
Conversion Factors: 1 MeV=1.6022×10−13 J. Since 1 amu is equivalent to 931.5 MeV of energy, the BE can be calculated using Equation 8.6.
What is the value of 1 amu?
In imprecise terms, one AMU is the average of the proton rest mass and the neutron rest mass. This is approximately 1.67377 x 10 -27 kilogram (kg),...
What is 1 u or 1 amu?
One atomic mass unit (1u) is a mass unit equal to exactly one-twelfth (1/12th) the mass of one atom of carbon-12 isotope.
What unit is used for amu?
atomic mass unitscalculation of atomic mass Atomic weight is measured in atomic mass units (amu), also called daltons. See below for a list of chem...
How many joules are in a proton?
133,000 JoulesEach proton will have energy of 8 TeV, so the energy of each bunch of protons is ~ 8*1011 TeV, i.e., 133,000 Joules (or 133 kilo Joul...
How do you convert J to Mol?
Re: How to convert kJ/mol to J Answer: Cancel out the 1/mol unit by dividing by the Avogadro constant. Then convert kJ to J by multiplying the kJ value by 1000 (because of the conversion factor 1 kJ = 1000 J).
How do you convert kJ mol to J photon?
A slightly different way would be to use Eλ = hc (with the wavelength in meters) and solve for E, then multiply the answer times Avogadro's Number. Finally, divide by 1000 to get kJ/mol. Example #3: How many kJ/mol (remember: mol means mole of photons) of energy is contained in light with a wavelength of 496.36 nm?
What do you mean by 1 amu?
An atomic mass unit (symbolized AMU or amu) is defined as precisely 1/12 the mass of an atom of carbon-12. The carbon-12 (C-12) atom has six protons and six neutrons in its nucleus. In imprecise terms, one AMU is the average of the proton rest mass and the neutron rest mass.
How do you find the value of one AMU?
Believe it or not, mathematically, moles and Avogadro's constant bring us back to atomic mass units. Dividing both sides by Avogadro's constant, we find that 1 amu is equal to 1.66 * 10^-27 kg. This means that atomic mass units can be easily converted to kilograms or grams. For example, carbon-12 has a mass of 12 amu.
What do you understand by 1 amu or 1u?
1-An atomic mass unit (u) is a unit of mass used to express atomic and molecular weights. One atomic mass unit (1u) or 1 a.m.u. is defined as one twelfeth (1/12) of the mass of an atom of carbon-12.
How much AMU is an electron?
Protons, neutrons, and electrons: Both protons and neutrons have a mass of 1 amu and are found in the nucleus. However, protons have a charge of +1, and neutrons are uncharged. Electrons have a mass of approximately 0 amu, orbit the nucleus, and have a charge of -1.
How do you find energy equivalent?
This formula states that the equivalent energy (E) can be calculated as the mass (m) multiplied by the speed of light (c = ~3×108 m/s) squared. Similarly, anything having energy exhibits a corresponding mass m given by its energy E divided by the speed of light squared c2.
What is the unit of energy of an atom?
An atomic mass unit, or amu, is one-twelfth of the mass of an unbound atom of carbon-12, and it used to express the mass of atomic and subatomic particles. The joule is the unit of energy in the International System of Units.
Why do we use full accuracy in mass measurements?
In calculating the mass defect use the full accuracy of mass measurements, because the difference in mass is small compared to the mass of the atom.
Unified Atomic Mass Units to Joules Conversion
u stands for unified atomic mass units and J stands for joules. The formula used in unified atomic mass units to joules conversion is 1 Unified Atomic Mass Unit = 1.4924179527347E-10 Joule. In other words, 1 unified atomic mass unit is 6700535854 times smaller than a joule.
Convert Unified Atomic Mass Unit to Joule
How to convert unified atomic mass unit to joule? In the energy measurement, first choose unified atomic mass unit from the left dropdown and joule from the right dropdown, enter the value you want to convert and click on 'convert'.
Unified Atomic Mass Units to Joules Converter
Units of measurement use the International System of Units, better known as SI units, which provide a standard for measuring the physical properties of matter. Measurement like energy finds its use in a number of places right from education to industrial usage.
What is the unit of energy?
In physics it is defined as the property of objects or fields, which allows it to perform work on other objects, for example to cause motion. The SI unit for energy is a joule. One joule represents the amount of energy expended while applying a force of 1 newton to a body and moving it for one meter.
How does biofuel work?
Biomass or biofuel generates energy when plant material is burned. During this process solar energy that plants generated through photosynthesis is released as heat. It is widely used in everyday life, for example to provide warmth for heating and cooking, and also as fuel for transportation. Alcohols and oils can be made from plants, and animal fat-based biofuel is also in use. One variation of biofuel, biodiesel, is used in the automotive industry both as an additive to other diesel fuels, or by itself.
How is nuclear energy generated?
It is generated through a controlled nuclear fission reaction,where a nucleus of an atom splits into smaller parts and releases energy . The energy heats water and produces steam, which, in turn, moves the turbines.