How do you calculate gram solubility?
- Measure 100 mL of distilled water and pour into a clean, empty beaker or jar.
- Use the kitchen balance to weigh out the suggested amount of the solute to be tested.
How do you calculate grams per 100 ml solubility?
Calculating gram per 100 mL solubility, given the Ksp. Return to Equilibrium Menu. Basically, this type of problem will (1) calculate the molar solubility from the Ksp. Then, in an additional step, (2) calculate grams per liter from moles per liter. From there, it is an easy third step to g/100mL.
What is solubility and how is It measured?
Since the solubility is temperature-dependent there should be uniform temperature throughout the system when dissolving substances. Solubility is measured either in grams per 100 g of solvent g/100g or number of moles per 1 L of the solution Formula to calculate solubility.
How do you find the solubility product of an ionic compound?
Step 1: Find the dissociation equation for the compound and identify the ions in the solution. Step 2: Set up the solubility product constant equation with the ions identified in Step 1. $$K_ {sp} = [Ag^ {1+} ]^ {2} [S^ {2-} ] $$
How do you find the solubility of NaNO3?
Divide the mass of the compound by the mass of the solvent and then multiply by 100 g to calculate the solubility in g/100g . Solubility of NaNO3=21.9g or NaNO3 x 100 g/ 25 g =87.6. Calculate the molar mass of the dissolved compound as the sum of mass of all atoms in the molecule. Click to see full answer.
How do you calculate how many grams to dissolve in water?
5:577:58Calculating how much Solute will Dissolve - YouTubeYouTubeStart of suggested clipEnd of suggested clipIf you try to dissolve 150 grams of ammonium chloride in 200 grams of water at 70 degrees CelsiusMoreIf you try to dissolve 150 grams of ammonium chloride in 200 grams of water at 70 degrees Celsius then how much will remain undissolved.
How do you find grams given KSP?
1) multiply the g/100mL value by 10/10. This converts it to grams per 1000 mL or, better yet, grams per liter. (Sometimes the data is given in g/L.
How do you calculate solubility product?
In this case, we calculate the solubility product by taking the solid's solubility expressed in units of moles per liter (mol/L), known as its molar solubility. The concentration of Ca2+ in a saturated solution of CaF2 is 2.1 × 10–4 M; therefore, that of F– is 4.2 × 10–4 M, that is, twice the concentration of Ca2+.
What does g mean in solubility?
Solubility is generally expressed as the number of grams of solute in one liter of saturated solution. For example, solubility in water might be reported as 12 g/L at 25 oC. Molar solubility is the number of moles of solute in one liter of saturated solution.
How do you calculate solubility with KSP?
0:152:55How to Calculate Solubility from Solubility Product - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo my ksp will be a g plus concentration which is x b r minus concentration which is also x soMoreSo my ksp will be a g plus concentration which is x b r minus concentration which is also x so therefore this would be an x squared.
How do you calculate solubility from grams per liter from KSP?
3:417:55Ksp to molar solubility or g/L - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo what we're going to do then is we're going to take this value left to point one times 10 to theMoreSo what we're going to do then is we're going to take this value left to point one times 10 to the negative fourth. Moles per liter and we are going to convert this to grams.
How do you calculate solubility Using Henry's Law?
1:054:3215 5e Using Henry's Law to calculate the solubility of a gas - YouTubeYouTubeStart of suggested clipEnd of suggested clipTimes the pressure of the gas. So the concentration of the gas equals enter is our constant timesMoreTimes the pressure of the gas. So the concentration of the gas equals enter is our constant times the pressure of the gas. Note that the Henry's law constant. Changes which change in temperature.
What is unit of solubility?
The unit of solubility is generally in mg/L (milligrams per liter) or ppm (parts per million).
Is solubility the same as concentration?
Solubility is typically a limit to how much solute can dissolve in a given amount of solvent. Concentration is the quantitative amount of solute dissolved at any concentration in a solvent.
What does g 100ml mean?
Therefore, 100 g of water will have a volume of 100 × 1 mL = 100 mL at 25°C. So at 25°C and 101.3 kPa, the solubility of a solute in water given as mass in grams per 100 g water is the same as the solubility of the solute given as mass in grams per 100 mL of water.
How do you use Table g in chemistry?
4:268:10Solutions: Table G (Solubility Curves) - YouTubeYouTubeStart of suggested clipEnd of suggested clipIf you're asked about a certain number of grams of solute at a given temperature and you do yourMoreIf you're asked about a certain number of grams of solute at a given temperature and you do your Cartesian plot go over to temperature up to that number of grams.
How do you find Delta g from solubility?
Therefore, from the equation ΔG = ΔH - TΔS we should predict that the solubility of every compound should increase with increasing temperature. That prediction turns out to be correct for nearly every solvent and solute.
Calculating the Solubility
Step 1: Find the dissociation equation for the compound and identify the ions in the solution.
Calculating the Solubility Vocabulary
Solubility: the maximum amount of a substance that will dissolve in a given volume of solvent at a given temperature and pressure.
Calculating the Solubility Example
Calculate the solubility of silver sulfide, Ag_ {2}S if its solubility product constant {eq}K_ {sp} {/eq} is {eq}8 \times 10^ {-51} {/eq} at room temperature.
Calculating the Solubility Example
Calculate the solubility of ferric oxyhydroxide, Fe (OH)_ {3} if its solubility product constant {eq}K_ {sp} {/eq} is {eq}1.6 \times 10^ {-39} {/eq} at room temperature.
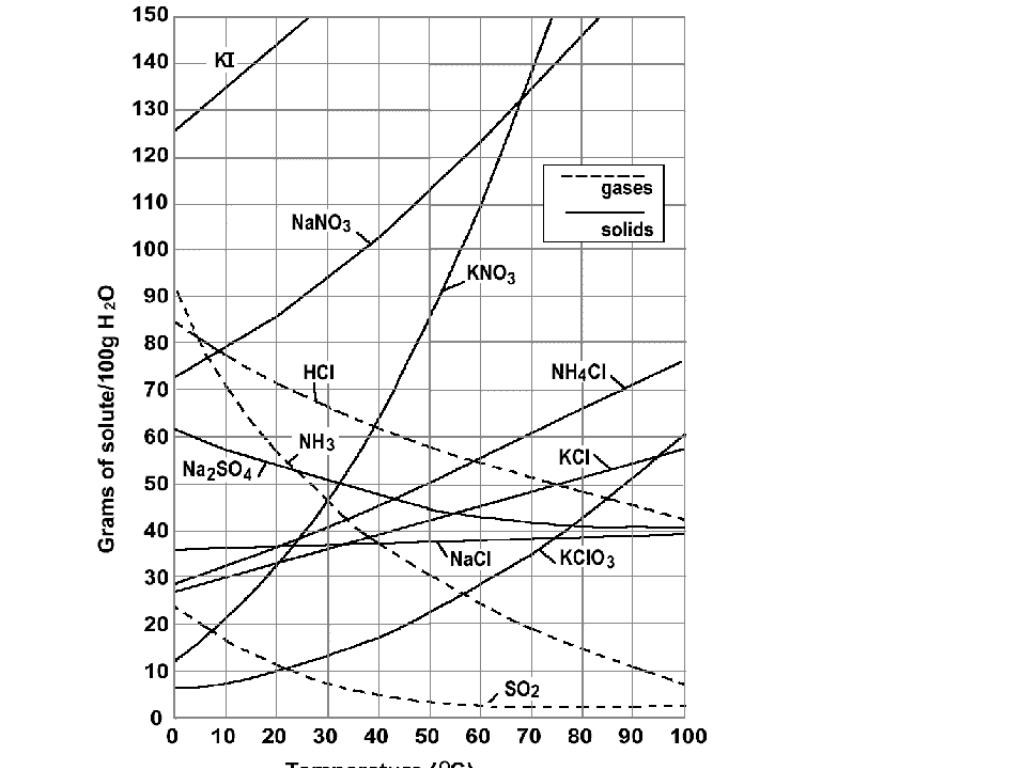
What is the solubility curve?
Solubility curves show how the solubility of a solute in a given solvent changes as the temperature changes.
How much NaCl can be dissolved in 100 g of water?
At 20°C (about room temperature), dissolving 35.89 g of NaCl ( s) in 100 g of water produces a saturated solution.
What happens to a supersaturated solution of a solid dissolved in water?
For a supersaturated solution of a solid dissolved in water, the solid (crystals or precipitate) will spontaneously form to reduce the mass of solute dissolved in the solution until the solution becomes saturated . We can use the solubility curve to predict the mass of solid that should precipitate (or crystallise) out of solution.
What is the maximum mass of a solute that can be dissolved in a given mass of solvent at
Remember that solubility refers to the maximum mass of solute that can be dissolved in a given mass of solvent at a specified temperature. In this case the solute is sodium chloride (NaCl (s)) and the solvent is 100 g of water.
What temperature does NaCl dissolve in?
Consider point A, 37 g of NaCl (s) will dissolve in 100 g of water at 80°C.
Why can't you use the volume of water to determine concentration?
This means that you can't use the volume of water to determine concentration if you are going to the change the temperature because the volume of the water also changes with temperature! Solubility data are therefore given as a ratio of the mass of solute to a fixed mass of solvent.
Does sodium chloride increase solubility?
Different substances will have different solubility curves. For lots of salts, like sodium chloride, the solubility of the salt in water increases with increasing temperature. But for gases and some other salts, like lithium sulfate, the solubility decreases as the temperature increases.
Calculating The Solubility
- Step 1:Find the dissociation equation for the compound and identify the ions in the solution. For example, a compound {eq}C{/eq} that dissolves into ions {eq}A{/eq} and {eq}B{/eq} can be written as: $$C \rightarrow aA + bB$$ Step 2: Set up the solubility product constant equation with the ions identified in Step 1so that the given {eq}K_{sp}{/eq} i...
Calculating The Solubility Vocabulary
- Solubility:the maximum amount of a substance that will dissolve in a given volume of solvent at a given temperature and pressure. Solubility product constant:{eq}K_{sp}{/eq}, the equilibrium constant for the dissolving of an ionic compound in water. Let's practice calculating solubility with the following two examples.
Calculating The Solubility Example
- Calculate the solubility of silver sulfide, Ag_{2}S if its solubility product constant {eq}K_{sp}{/eq} is {eq}8 \times 10^{-51}{/eq} at room temperature. Step 1:Find the dissociation equation for the compound and identify the ions in the solution. $$Ag_{2}S \rightarrow 2Ag^{1+} + S^{2-}$$ Step 2: Set up the solubility product constant equation with the ions identified in Step 1. $$K_{sp} = [Ag^…