How do you balance c3h6 o2 co2 h2o?
- Write down your given equation.
- Write down the number of atoms per element.
- Save hydrogen and oxygen for last, as they are often on both sides.
- Start with single elements.
- Use a coefficient to balance the single carbon atom.
- Balance the hydrogen atoms next.
- Balance the oxygen atoms.
How to balance C6H12O6+O2=CO2+H2O?
Since you have minimum 6 Carbons on the right, you need at least 6 CO2 to balance the Carbon, as CO2 has only 1 each. 6CO2 + H2O -----> C6H12O6 + O2 The Carbon is balanced, so let's try to balance the Hydrogen now. Since there are 12 Hydrogens on the right, we need 6 H2O to balance, as there are 2 Hydrogens in each.
What type of reaction is C6H6 O2 CO2 H2O?
C6H6 + O2 = CO2 + H2O This is The equation for the composition of Benzene. of Oxygen in O2 = 0 Heat is produced, light is emitted, or a precipitate is formed this is an indication of this. 2C6H6 + 15O2 --> 12CO2 + 6H2O.
How do you balance the equation C O2 CO CO2?
- 2CO + O2 =====> 2CO2
- When Carbon is burnt with insufficient supply of Oxygen Carbon monoxide will be formed.
- This emission of carbon monoxide from the vehicles makes the air toxic or poisonous.
- Effects of Carbon monoxide poisoning:
- Carbon monoxide poisoning is caused by inhaling combustion fumes. ...
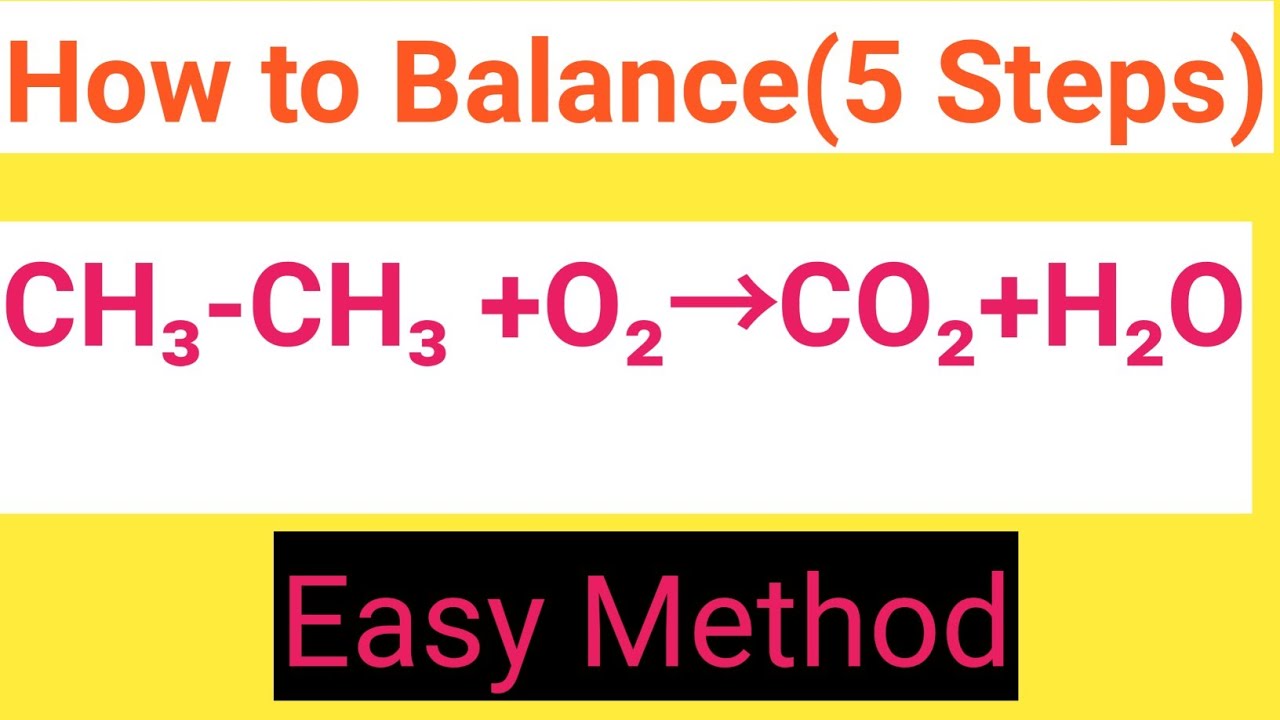
How to balance C2H6?
- Check that the reaction is correct - in this case it is.
- Start at the front with C. 2 on the left, 1 on the right, so you need 2CO2. ...
- That leaves the Os; 2 on the left and 7 on the right. So you would need 3.5O2 on the left. ...
- Final check: Left - 4 C, 12 H and 14 O; Right 4 C, 12 H and 14 O. Balanced.
How to balance a single carbon atom?
Method 1 Doing a Traditional Balance. Write down your given equation. Write down the number of atoms per element. Save hydrogen and oxygen for last, as they are often on both sides. Start with single elements. Use a coefficient to balance the single carbon atom. Balance the hydrogen atoms next. Balance the oxygen atoms.
What is the equation for C6H12O6?
C6H12O6 + O2 = CO2 +H2O, I first count the carbon atoms on both sides. There are 6 in C6H12O6, on the left side, so I write a 6 before CO2 to have 6 atoms of carbon on the other side. C6H12O6 + O2 = 6CO2 + 6H2O so far.
What gives carbon dioxide and water?
We know that complete combustion of hydrocarbons or oxyhydrocarbons, as alcohols, ethers, or aldehydes, or acids, gives carbon dioxide and water …. And so …
How to keep oxygen in oxidation reaction?
In the oxidation reaction indicated, keep it simple by initially focusing on the number of C atoms that end up as CO2. Also keep the oxygen as atoms. You can always double up to molecular O2 once you have achieved a balance.
How many mols of oxygen does butane react with?
This equation is valid on a molar level (One mole butane reacts with six- and-a- half mols of oxygen gas to give four mols carbon dioxide and five mols water.
What does z = 5 equate to?
from which it is evident that z = 5 to equate to the H10 of the C5H10.
How many atoms of Ca are needed for O2?
So, at least 2 atoms of ‘Ca’ are needed for 1 molecule of ‘O2’ for the reaction to complete and the system to be stable.
Does H appear in only one reactant?
In this case, H does appear in only one reactant and one product.
Is CaO unstable?
Here, after forming one molecule of CaO, ‘Ca’ is exhausted, but one atomic part of oxygen remains in the system, uncombined, which makes the system highly unstable.
What is the reaction between C4H4 and 2H2?
C4H4 (g) + 2 H2 (g) --> C4H8 (g) Combustion reactions involve reacting a substance with oxygen. When compounds containing carbon and hydrogen are combusted, carbon dioxide and water are the products. Using the enthalpies of
What is the heat of urea?
Solid urea, (NH2)2CO, burns to give CO2,N2, and liquid H2O. Its heat of combustion is -632.2 kJ/mol. Balance the equation for combustion of urea, and calculate the heat generated per mole of H2O formed. (Enter in kJ.)