How are complete ionic equations and net ionic equations different from chemical equations? Your complete ionic equation includes all ions in solution, including spectator ions. Your net ionic equation leaves out spectator ions and focuses on what changes in the reaction.
How do you write a complete ionic equation?
To write the complete ionic equation:
- Start with a balanced molecular equation.
- Break all soluble strong electrolytes (compounds with (aq) beside them) into their ions. indicate the correct formula and charge of each ion. indicate the correct number of each ion.
- Bring down all compounds with (s), (l), or (g) unchanged.
How to write a complete ionic equation?
Writing a Net Ionic Equation: Physical State Identifiers Are Provided
- Look at the molecular equation and identify which species are in the aqueous state. ...
- Write the complete ionic equation by breaking the aqueous species into their constituent ions. ...
- Cross out the spectator ions on both sides. ...
- Rewrite the equation without the spectator ions. ...
What are the rules for writing an ionic equation?
Writing Net Ionic Equations
- Only consider breaking up the (aq) substances .
- Only break up strong electrolytes .
- Delete any ions that appear on both sides of the equation.
How do you calculate an ionic equation?
Part 2 Part 2 of 2: Writing a Net Ionic Equation
- Balance the complete molecular equation. Before writing a net ionic equation, you must first make sure your starting equation is completely balanced.
- Identify the states of matter of each compound in the equation. ...
- Determine what species will dissociate (separate into cations and anions) in solution. ...
- Calculate the charge of each dissociated ion. ...
What is the difference between chemical equations and net ionic equations?
The key difference between Complete ionic and net ionic equation is that complete ionic equation gives all the ionic species participated in the chemical reaction whereas net ionic reaction gives the chemical species participated in the formation of the final product.
What is the difference between a complete ionic equation and net ionic equation?
In the complete ionic equation, soluble ionic compounds and strong acids are rewritten as dissociated ions. In the net ionic equation, any ions that do not participate in the reaction (called spectator ions) are excluded.
What is a complete ionic equation?
The complete ionic equation indicates all of the dissociated ions in a chemical reaction. The net ionic equation cancels out ions that appear on both sides of the reaction arrow because they essentially don't participate in the reaction of interest. The ions that are canceled out are called spectator ions.
What is the difference between molecular complete ionic and net ionic equation in which circumstances would the complete and net ionic equation of a reaction is identical?
Net ionic equation shows only the chemical species that are involved in a reaction. Complete ionic equation shows the chemical species that are involved in a reaction and the spectator ions. The complete and net ionic equations will be identical if there are no spectator ions.
What is a net ionic equation in chemistry?
The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical reaction.
What is the advantage of a net ionic equation over a more typical full chemical equation?
The advantage of net ionic equations is that they show only those species that are directly involved in the reaction.
What is a net ionic equation quizlet?
Net ionic equations are equations that show only the soluble, strong electrolytes reacting (these are represented as ions) and omit the spectator ions, which go through the reaction unchanged.
Why do we write net ionic equations?
Net Ionic Equations Are Important The reason to write a chemical equation is to express what we believe is actually happening in a chemical reaction. One of the most useful applications of the concept of principal species is in writing net ionic equations.
What is a complete ionic equation apex?
Complete ionic equation is a chemical equation that explains the chemical reaction clearly indicating the ionic species present in a solution. The net ionic equation is a chemical equation which gives the ions that are participated in the formation of the final product.
How are complete ionic and net ionic equations written for chemical reactions in aqueous solutions?
Write all the soluble reactants and products in their dissociated form to give the complete ionic equation; then cancel species that appear on both sides of the complete ionic equation to give the net ionic equation.
How do you write a net ionic equation for an acid base reaction?
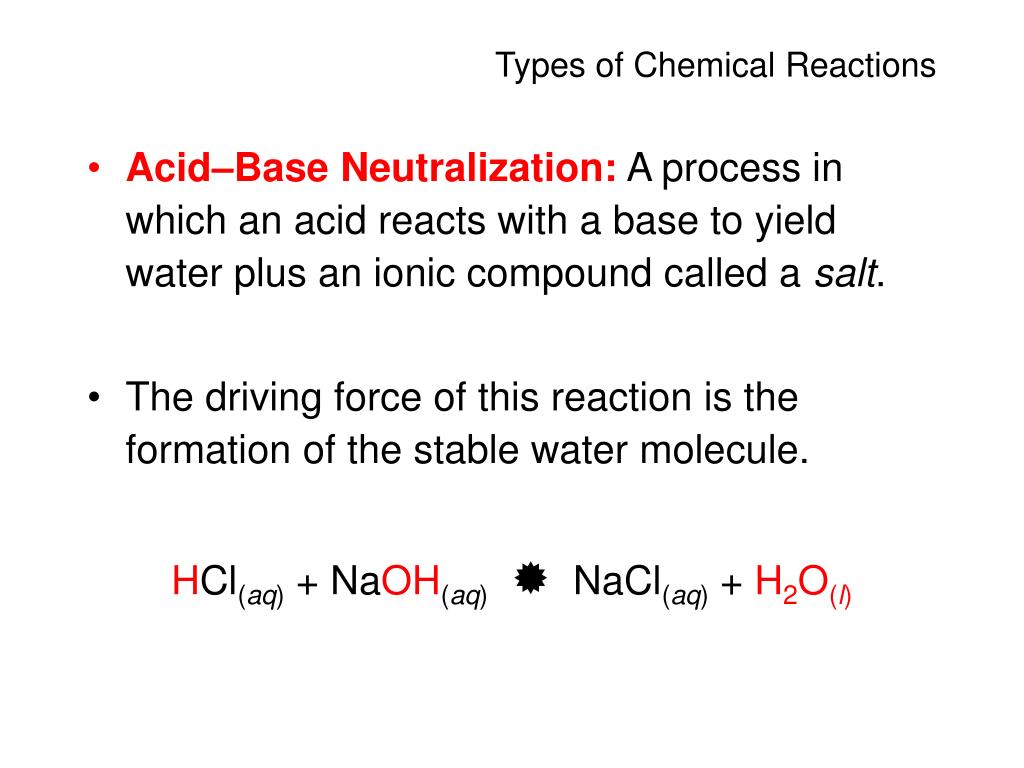
4:2513:33Acid Base Neutralization Reactions & Net Ionic Equations - ChemistryYouTubeStart of suggested clipEnd of suggested clipSo the final net ionic equation is h plus plus oh minus produces liquid water.MoreSo the final net ionic equation is h plus plus oh minus produces liquid water.
What is an ionic equation explain with an example?
An ionic equation is a chemical equation where the electrolytes in aqueous solution are written as dissociated ions. Example: 1) Sodium chloride(aq) + silver nitrate(aq) → silver chloride(s) + sodium nitrate(aq) >>Ag+(aq) + Cl-(aq) → AgCl(s) 2) Sodium(s) + hydrochloric acid(aq) -> sodium chloride(aq) + hydrogen(g).
What is the difference between a complete ionic equation and a net ionic equation?
A net ionic equation shows only the chemical species that are involved in a reaction, while a complete ionic equation also includes the spectator ions. We can find the net ionic equation for a given reaction using the following steps:
How to find the net ionic equation?
A net ionic equation shows only the chemical species that are involved in a reaction, while a complete ionic equation also includes the spectator ions. We can find the net ionic equation for a given reaction using the following steps: 1 Write the balanced molecular equation for the reaction, including the state of each substance. 2 Using your knowledge of solubility rules, strong acids, and strong bases, rewrite the molecular equation as a complete ionic equation that shows which compounds are dissociated into ions.#N#[I haven't learned about strong acids and bases yet!] 3 Identify and cancel out the spectator ions (the ions that appear on both sides of the equation).#N#[Want to double check your work?]
What is the net ionic equation for silver chloride?
This net ionic equation tells us that solid silver chloride is produced from dissolved and ions, regardless of the source of these ions. In comparison, the complete ionic equation tells us about all of the ions present in solution during the reaction, and the molecular equation tells us about the ionic compounds that were used as the sources ...
How does sodium interact with water?
Each chloride ion is interacting with multiple water molecules through the positive dipole of the water, and each sodium ion is interacting with water molecules through the negative dipole of the water.
What happens when ions are involved in a reaction?
When ions are involved in a reaction, the equation for the reaction can be written with various levels of detail. Depending on which part of the reaction you are interested in, ...
What are ionic species that remain unchanged during a reaction called?
Ionic species that remain unchanged like this during a reaction are called spectator ions. Since spectator ions appear on both sides of the equation, we can cancel them out, similar to how we can cancel out equal terms on either side of a math equation:
What is a balanced equation?
A molecular equation is sometimes simply called a balanced equation. In a molecular equation, any ionic compounds or acids are represented as neutral compounds using their chemical formulas. The state of each substance is indicated in parentheses after the formula.
What is net ionic equation?
In the net ionic equation, any ions that do not participate in the reaction (called spectator ions) are excluded. As a result, the net ionic equation shows only the species that are actually involved in the chemical reaction. Created by Sal Khan. This is the currently selected item.
What are the neutral molecules in the molecular equation?
Transcript. In the molecular equation for a reaction, all of the reactants and products are represented as neutral molecules (even soluble ionic compounds and strong acids). In the complete ionic equation, soluble ionic compounds and strong acids are rewritten as dissociated ions.
Why doesn't NaNO3 bond?
Direct link to von luger's post “They don't bond together ...”. They don't bond together here because the compound NaNO3 is soluble in water. In net ionic equations, only the formation of the precipitate is shown, not the soluble compound. You can check if a substance is soluble in water by using a solubility chart.
Can you decompose NaCl?
No, we can't call it decomposition because that would suggest there has been a chemical change. If you dissolve crystals of NaCl in water, you get a solution of Na+ and Cl- ions, but if you evaporate the water you get back your crystals of NaCl - overall, you've gone through a cycle and nothing has changed.
Why is there no net ionic equation?
There is no net ionic equation because there is no reaction (all fours ions are exactly the same on each side, all of them are stricken out as being spectator ions). Often NR is used to signify no reaction. You will find additional NR examples at 13, 23, 28, and 44 in my problem sets. There are several examples at #44.
What are ionic compounds?
Ionic compounds are between metals and nonmetals or between metals and polyatomic ions. Examples are sodium chloride (NaCl), magnesium nitrate [Mg (NO 3) 2] and ammonium sulfate [ (NH 4) 2 SO 4 ]. When an ionic substance is dissolved in aqueous solution, it ALWAYS ionizes and the ions always have a charge.
What is the reaction between sodium sulfate and barium chloride?
Here are two examples: barium chloride solution reacts with sodium sulfate solution to make solid barium sulfate and aqueous sodium chloride. aqueous solutions of hydrochloric acid and sodium hydroxide react to produce sodium chloride and water. The wording in problems like the above can vary somewhat.
Which ion is the spectator ion?
In both of the example equations above, the sodium ion and the chloride ion are the spectator ions. Here's why identifying the spectator ions is important: to go from a complete ionic equation to a net ionic equation, all spectator ions are eliminated from the equation.
Do molecules ionize in solution?
Molecular substances never ionize in solution, they exist as complete molecules and they NEVER have a charge. By the way, molecular compounds are also called covalent compounds. All the bonds in molecules like the examples above are covalent. Not one ionic bond exists in a covalent compound.
Can you use words for ionic equations?
Only full formulas (never words or ions) are involved in a complete molecular equation. Ions (never words) will be used for the complete ionic equation and the net ionic equation, which will follow just below. You can use words for an ionic equation (either complete or net), it just isn't usually done.
What is an ionic equation?
Similar to a molecular equation, which expresses compounds as molecules, an ionic equation is a chemical equation in which the electrolytes in aqueous solution are expressed as dissociated ions. Usually, this is a salt dissolved in water, where the ionic species are followed by ...
What are the two most common forms of ionic equations?
The two most common forms of ionic equations are complete ionic equations and net ionic equations. The complete ionic equation indicates all of the dissociated ions in a chemical reaction. The net ionic equation cancels out ions that appear on both sides of the reaction arrow because they essentially don't participate in the reaction of interest.
How are ions stabilized in aqueous solutions?
The ions in aqueous solutions are stabilized by ion-dipole interactions with water molecules. However, an ionic equation may be written for any electrolyte that dissociates and reacts in a polar solvent. In a balanced ionic equation, the number and type of atoms are the same on both sides of the reaction arrow.
Why are strong acids written as ions?
Strong acids, strong bases, and soluble ionic compounds (usually salts) exist as dissociated ions in aqueous solution, so they are written as ions in the ionic equation. Weak acids and bases and insoluble salts are usually written using their molecular formulas because only a small amount of them dissociates into ions.