How are anions formed from neutral atoms?
When a neutral atom gains one or more electrons, the number of electrons increases while the number of protons in the nucleus remains the same. The result is that the atom becomes an anion—an ion with a net negative charge. Click to see full answer. Also asked, how are anions formed?
How are cations and anions formed?
How are cations and anions formed from neutral atoms? 1 The electronic configuration of many ions is that of the closest noble gas to them in the periodic table. 2 An anion is an ion that has gained one or more electrons, acquiring a negative charge. 3 A cation is an ion that has lost one or more electrons, gaining a positive charge. More ...
Why do non-metals usually form anions?
Non-metals usually form anions. These are electron-rich species; hence they possess a net negative charge. Anions are generally bigger than their parent atom or molecule because the excess electrons suffer repulsion and contribute to the physical size of the electron cloud.
Can we predict whether an atom will form a cation or anion?
It can be possible to predict whether an atom will form a cation or an anion based on its position on the periodic table. Halogens always form anions, alkali metals and alkaline earth metals always form cations.
How cations and anions are formed?
Metallic atoms hold some of their electrons relatively loosely. Consequently, they tend to lose electrons and form cations. Conversely, most nonmetallic atoms attract electrons more strongly than metallic atoms, and so gain electrons to form anions.
How are neutral atoms converted into anion?
Neutral atoms tend to increase in size down a group and decrease across a period. When a neutral atom gains or loses an electron, creating an anion or cation, the atom's radius increases or decreases, respectively.
When a cation is formed from a neutral atom?
Cations are formed when a neutral atom loses an electron. Metals are prone to losing electrons as a result of the arrangement of electrons around the nucleus. Electrons occupy different orbitals around the nucleus, and these can be grouped into different energy levels.
What must happen in order to convert an atom into an cation?
An atom becomes an Ion (a) if it gains one or more electron(s) or (b) if it loses one or more electron(s). When it gains electrons it becomes negatively charged and is called an anion. When it loses electron(s) it becomes positively charged and is called a cation.
What is the process of a neutral atom acquiring either a positive or negative charge?
Ionization, or Ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule is called an ion.
How are cations formed?
Cations form when an atom loses one or more electrons. The resulting cation has the electron configuration of the noble gas atom in the row above it in the periodic table.
How is a cation formed explain with examples?
Cations are positively charged ions. They are formed when a metal loses its electrons. They lose one or more than one electron and do not lose any protons. Therefore, they possess a net positive charge.
What is cations and anions?
Cations are positively-charged ions (atoms or groups of atoms that have more protons than electrons due to having lost one or more electrons). Anions are negatively-charged ions (meaning they have more electrons than protons due to having gained one or more electrons).
Ions
An atom comprises a dense nucleus which is made up of positively charged protons and neutral neutrons surrounded by negatively charged electrons. An atom is electronically neutral if it has the same number of protons and electrons.
Cation
A cation is an atom or a group of atoms with more protons than electrons, consequently giving it a net positive charge.
Anion
An anion is an atom, or a group of atoms with more electrons than protons, consequently giving it a net negative charge.
Zwitter Ions
These ions are neutral and possess both positive and negative charges at different locations throughout the molecule. Amino acids are the most common example of zwitterions. They are made up of an ammonium or amino group that contains a positive charge and a carboxyl group that contains a negative charge.
Summary
When one or more electrons are removed from a neutral atom, a positive ion (cation) is formed. When one or more electrons attach themselves to neutral atoms, a negative one (anion) is formed.
Frequently Asked Questions (FAQs) on Cations and Anions
Q.1. Between anions or cations, which one is bigger? Ans: Anions are larger, whereas cations are smaller than their corresponding neutral atom. This is because the addition of electrons increases the electron-electron repulsion, increasing the electron cloud. Whereas, removal of electrons results in decreased repulsion between electrons.
Which element can form both cations and anions?
However, some elements are capable of forming both cations and anions given the right conditions. One example is hydrogen , which may gain (H -) or lose (H +) an electron, forming hydride compounds such as ZnH 2 (where it is an anion) and hydron compounds such as H 2 O (where it is a cation).
When atoms from a metallic and a nonmetallic element combine, do they form ions?
Therefore, when atoms from a metallic and a nonmetallic element combine, the nonmetallic atoms tend to draw one or more electrons away from the metallic atoms to form ions . These oppositely charged ions then attract one other to form ionic bonds and produce ionic compounds with no overall net charge.
What are the ionic properties of batteries?
Ionic properties are central to the function of batteries too. Batteries have two electrodes made of conductive material, the cathode which is the positive end where the electrical current leaves/electrons enter, and the anode where the electrical current enters/ electrons leave.
How many electrons does chlorine gain?
The number of electrons gained, and so the charge of the ion, is indicated after the chemical symbol, e.g. chlorine (Cl) gains one electron to become Cl-, whilst oxygen (O) gains two electrons to become O2-.
What are ionic properties?
Ionic properties are central to the function of batteries too .
How many electrons do cations lose?
For a cation to form, one or more electrons must be lost, typically pulled away by atoms with a stronger affinity for them. The number of electrons lost, and so the charge of the ion, is indicated after the chemical symbol, e.g. silver (Ag) loses one electron to become Ag +, whilst zinc (Zn) loses two electrons to become Zn 2+.
What is it called when an atom is not balanced?
However, if they are not balanced, they will be charged. These charged species are called ions .
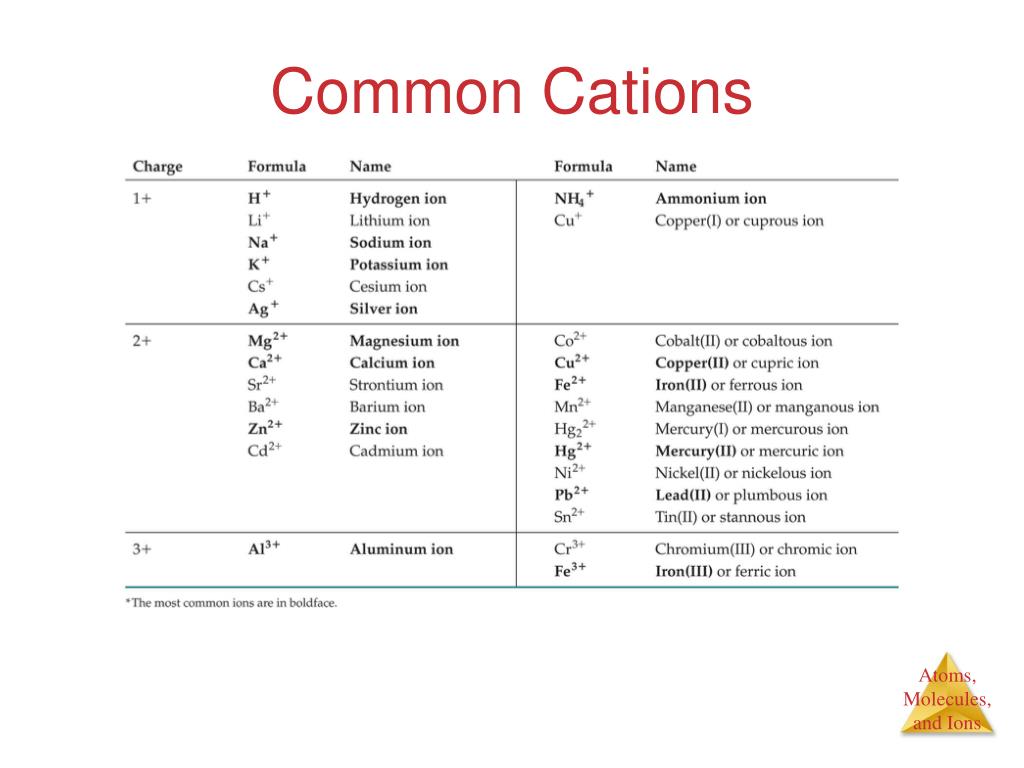
Ions
Cation
- A cation is an atom or a group of atoms with more protons than electrons, consequently giving it a net positive charge. Formation of cation Cations are formed- 1. When there is a removal or loss of electrons from neutral atoms or other molecules. 2. By the combination of positive ions with other molecules. 3. By the cleavage of the covalent bond causes the shared pair of electrons to be ass…
Anion
- An anion is an atom, or a group of atoms with more electrons than protons, consequently giving it a net negative charge. Formation of anion Anions are formed- 1. When there is an addition or gain of electrons to neutral atoms or other molecules. 2. By the combination of negative ions with other molecules. 3. By the cleavage of a covalent bond causes the shared pair of electrons to b…
Zwitter Ions
- These ions are neutral and possess both positive and negative charges at different locations throughout the molecule. Amino acids are the most common example of zwitterions. They are made up of an ammonium or amino group that contains a positive charge and a carboxyl group that contains a negative charge.
Summary
- When one or more electrons are removed from a neutral atom, a positive ion (cation) is formed. When one or more electrons attach themselves to neutral atoms, a negative one (anion) is formed. The designations cation or anion come from the early experiments with electricity in which positively charged particles were attracted to the cathode (negativ...
Frequently Asked Questions (FAQs) on Cations and Anions
- Q.1. Between anions or cations, which one is bigger? Ans:Anions are larger, whereas cations are smaller than their corresponding neutral atom. This is because the addition of electrons increases the electron-electron repulsion, increasing the electron cloud. Whereas, removal of electrons results in decreased repulsion between electrons. Hence, anions are bigger, and cations are sm…