Key Takeaways
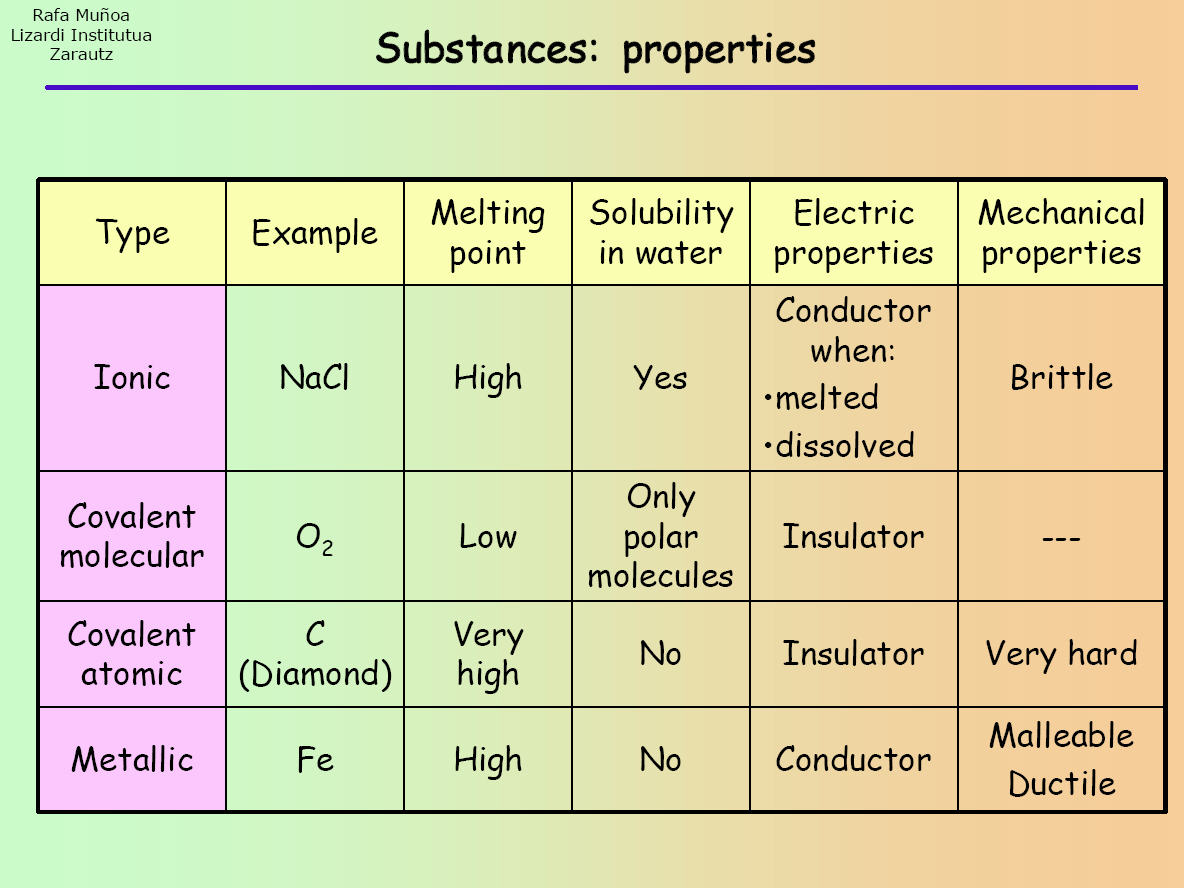
- Ionic compounds have high melting points.
- Ionic compounds are hard and brittle.
- Ionic compounds dissociate into ions when dissolved in water.
- Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not.
- An ionic compound can be identified by its chemical formula: metal + nonmetal or polyatomic ions.
What are 5 examples of ionic compounds?
Ionic bond examples include:
- LiF - Lithium Fluoride
- LiCl - Lithium Chloride
- LiBr - Lithium Bromide
- LiI - Lithium Iodide
- NaF - Sodium Fluoride
- NaCl - Sodium Chloride
- NaBr - Sodium Bromide
- NaI - Sodium Iodide
- KF - Potassium Fluoride
- KCl - Potassium Chloride
What are the main properties of ionic compounds?
Properties of ionic compounds: 1. Ionic compounds are solids & are somewhat hard because of strong force of attraction between the positive and negative ions. 2. Ionic compounds have high melting and boiling points. 3. Ionic compounds are generally soluble in water & insoluble in solvents, such as kerosene, petrol etc., 4.
Why are ionic compounds insoluble in water?
- I know it’s nothin special but I isolated strawberry DNA after seein a NileRed video on it. ...
- The two elements that are liquid at room temperature
- My Large Liquid Chlorine Sample.
- Chemistry is so fun when you understand what you’re doing. ...
- Zinc hydroxide in nitric acid ... ...
- Would smoking a bong filled with coffee get you caffeinated. ...
How do ionic compounds dissolve?
The reason why water is able to dissolve an ionic compound
- The water molecules begin to surround the ionic compound, and then start to move in. ...
- The water molecules then surround the loose ions in a process called hydration. ...
- The negatively charged oxygen dipoles in the water molecule attracts the positively charged sodium ions. ...
Do ionic compounds dissociate?
Under most conditions, ionic compounds will dissociate nearly completely when dissolved, and so they are classified as strong electrolytes.
Do most ionic compounds dissociate in water?
Most ionic compounds are soluble in water. Polar water molecules have a strong attraction for charged ions and the charged ions become solvated as they dissociate into the water and ionic compounds are soluble in water.
Do all ionic compounds dissociate in water?
There are notable exceptions: ionic compounds containing highly polarising ions (ones that are small and have a high charge) will usually not dissolve in water, but rather react with it, or just not dissolve at all. Oxides are the most common example.
Do ionic compounds dissolve or dissociate?
Ionic Compounds in Water When ionic compounds dissolve in water, they break apart into the ions that make them up through a process called dissociation.
Which ionic compounds do not dissolve in water?
Which ionic compounds are insoluble in water?Any ionic compound containing carbonate, oxide, or hydroxide anion is insoluble.Barium sulfate, calcium sulfate, and lead(II) sulfate are insoluble.
Is all ionic compounds soluble?
Absolutely not. A great many ionic species are INSOLUBLE in water.
What types of compounds never dissociate?
Most molecular substances do not dissociate in water. substances, such as CH3OH or O2, do not dissociate into ions in aqueous solution. The molecules remain intact. some monatomic ions from their positions in the periodic table.
Do covalent compounds dissociate in water?
- When covalent compounds are dissolved in water they dissociate into molecules, but not into ions. Water is a polar solvent, but covalent compounds are nonpolar. This implies that covalent compounds don't dissolve in water and make a separate layer on the surface of the water.
Why do some ionic materials not dissolve in water?
If the hydration energy of an ionic compound exceeds its lattice energy, the lattice is broken and the ions in the compound separate, causing the compound to dissolve. If the hydration energy of the compound is lesser than the lattice energy, the compound will not dissolve.
Do ionic bonds dissolve in water?
When you immerse an ionic compound in water, the ions are attracted to the water molecules, each of which carries a polar charge. If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound dissolves.
Are ionic compounds that dissolves to form ions in solution completely?
strong electrolytesUnder most conditions, ionic compounds will dissociate nearly completely when dissolved, and so they are classified as strong electrolytes.
What is the name of the reaction that occurs when ionic compounds dissociate?
In electrolytic or ionic dissociation, ionic compounds dissociate when put in water, going from their compound form to a positive ion and a negative ion. This type of dissociation reaction is called ionization. The reaction is called ionization and the resulting solutions are referred to as electrolytic solutions.
What is dissociation in chemistry?
Dissociation, in chemistry, is the breaking down of a compound substance into its simpler substances.
What is the difference between ionic and electrolytic dissociation?
Electrolytic or ionic dissociation is when compounds dissociate into a negative and positive ion. The resulting solutions are referred to as electrolytic solutions because they can conduct electricity. We'll see this clearly when you write the dissociation in formula form later. This type of dissociation reaction is called ionization.
How to tell if an ion is positive or negative?
Moving to the right side of the formula, you'll see (aq) after each ion. This tells you that these ions are existing in an aqueous solution after the dissociation. If water is the reactant, then you don't include the aq in parentheses. You also see superscripts of + and - to let you know which is the positive ion and which is the negative. It's very important to write these superscripts with your ions to tell them apart from their molecular forms.
What happens after dissociation of sodium chloride?
After dissociation, it breaks apart into a positive sodium ion and a negative chlorine ion.
Is dissociation a chemical reaction?
One thing to note here is that dissociations are reversible chemical reactions. All dissociations have just one reactant with multiple products. If water is the reactant though, don't include it.
What happens when ionic compounds dissolve?
When they dissolve, they are hydrated and release the free cations (+) & anions (-) into the aqueous solutions. The partially soluble ionic compounds also release these oppositely charged hydrated ions. The degree of ionisation depends on the degree of solubility. The solubility is also determined by other factors such as temperature, the volume of solvent, the mass of solute etc.
When ionic salts dissociate in aqueous solutions, the hydrated ions are free to?
When ionic salts dissociate in aqueous solutions, the hydrated ions are free to move about. However, their movement is random, and there is no charge separation that occurs - therefore, the aqueous solution is electrically neutral overall.
What is the solubility of ionic compounds?
So solubility is a balance between these two types of energy for each ionic compound. So there is a vast range of solubilities of ionic compounds.
Where do ions go in a battery?
Then - ions from solution go to + electrode (on the left electrode) then travel through wire to - electrode (on the right electrode). This - electrode is an excess of electrons. The + ions in water will come to this - electrode (right) and get electrons. The + ions become neutral molecules and stay in water. These electrons do travel through wire come from - ions in water to other - electrode. The battery has to be between. These electrons travel to its - electrode to feed to the+ ions in water then become neutral molecules and stay in solution.
What is the process of decomposition of ionic compounds?
Electrolysis is a chemical process in which ionic compounds, in their molten state or aqueous solutions are decomposed during the passage of electricity.
How do ionic compounds form?
Ionic compound generally form crystals at the sorts of temperatures we work at. So they are stabilized by the energies of +/- interactions in the crystal structure, which can be very strong. These energies are both attractive (+ -) and repulsive (++, - -). They depend on ion charge/size ratio and cation/anion size/number ratios. In many cases, the size/charge ratios can be adjusted by incorporating water into the lattice, usually bonded to the cations. However, NOT ALL ionic compounds are “aqueous” in that sense, some will actually lose water on standing in air (washing soda seems to ring a bell !)
What happens to electrons in water?
Electrons in water (solution) are always -ions then turn into neutral molecules.
Why do ionic bonds not dissociate?
Even an ionic bond would not dissociate if there was no driving force. Ionic bonds are formed because forming them releases energy when compared to naked atoms and/or ions in space. When dissolving ionic compounds in (say) water, the driving force is, in fact, the formation of coordination complexes between the solvent molecules and bothtypes of ions.
Why do ionic compounds dissolve?
So why do ionic compounds dissolve? Mostly due to the solvent-compound interactions. See more here.
What is a partially ionic and partially covalent bond called?
Bonds with partially ionic and partially covalent character are called polar covalent bonds. Nevertheless, ionic bonding is considered to be a form of noncovalent bonding. According to Coulomb's law equation ( 1), the force between two charges depend on the medium in which they are placed.
What is an ionic bond?
10. An ionic bond is the bonding between a non-metal and a metal, that occurs when charged atoms (ions) attract after one loses one or more of its electrons ,and gives it to the other molecule , for example sodium and chlorine. This makes the bond stronger and harder to break.
What is the difference between ionic and covalent bonds?
In other words, an ionic bond is the electrostatic force of attraction between two oppositely charged ions . The positive ion is called cation, and the negative ion is the anion. It is like the north and south poles of a magnet. A covalent bond is a chemical bond that involves the sharing of electron pairs between atoms.
What is a dipolar covalent bond?
A dipolar bond, also known as a dative covalent bond or coordinate bond is a kind of 2-center, 2-electron covalent bond in which the two electrons derive from the same atom. ...
What is covalent bonding?
A covalent bond is a chemical bond that involves the sharing of electron pairs between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding. For many molecules, the sharing of electrons allows each atom to attain the equivalent of a full outer shell, ...