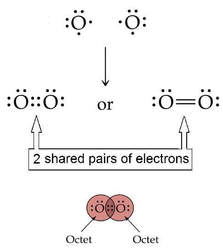
Why is there a double bond in oxygen?
- O=O double bonds are much stronger than S=S double bonds.
- S-S single bonds are almost twice as strong as O-O single bonds.
- Sulfur ( EN = 2.58) is much less electronegative than oxygen ( EN = 3.44).
- Sulfur can expand its valence shell to hold more than eight electrons, but oxygen cannot.
How can oxygen make two single bonds?
two single covalent bonds Which substance when combined with oxygen will form a covalent bond? Covalent bonds usually occur between nonmetals. For example, in water (H2O) each hydrogen (H) and oxygen (O) share a pair of electrons to make a molecule of two hydrogen atoms single bonded to a single oxygen atom.
Does oxygen contain a triple bond?
Oxygen tends not to form triple bonds due to formal charge reasons. If oxygen starts with 6 electrons and forms a triple bond, then it has 2 lone pair electrons. Using the formal charge formula, 6 – (2+6/2) = 1. Since oxygen is so electronegative, it’ll tend not to have a positive formal charge. Why does O2 not have a triple bond?
Is a triple bond stronger than a single bond?
Triple bonds are stronger than the equivalent single bonds or double bonds, with a bond order of three. The most common triple bond, that between two carbon atoms, can be found in alkynes. How do you know which bond is least polar?
See more
Can oxygen form 3 bonds?
Oxygen has 6 electrons in its outermost shell. However, if you give it a positive charge by removing an electron, then it would only have 5 electrons in its outermost shell. Therefore, it can now from 3 bonds while carrying a positive charge.
How many bond can oxygen form?
two singleOxygen is capable of forming two single bonds because on its outer shell it has six valence electrons. Instead of sacrificing all six to become stable, it is simpler for an oxygen atom to accept or share two electrons. Six valence electrons have an oxygen atom, like all elements in group 6A of the periodic table.
What elements can form triple bonds?
Carbon and nitrogen in particular can share up to three of their outer electrons with another atom of the same kind, forming triple bonds. These are stronger than single or double bonds and so result in some of the most stable molecules known.
Can oxygen and carbon form a triple bond?
The carbon monoxide molecule is correctly represented by a triple covalent bond between the carbon and oxygen atoms. One of the bonds is a coordinate covalent bond, a covalent bond in which one of the atoms contributes both of the electrons in the shared pair.
Can oxygen have 3 lone pairs?
Normally, oxygen atoms have 2 bonds and 2 lone pairs. Atoms of hydrogen have 1 bond and no lone pairs. Normally, oxygen atoms have 2 bonds and 2 lone pairs. Usually, chlorine atoms have one bond and three lone pairs.
Is oxygen a trivalent?
Oxygen is divalent while nitrogen is trivalent.
Can oxygen have four bonds?
This is false that oxygen atom can form four covalent bonds with up to four other atoms. this can be explained as: A covalent bond is formed between two atoms by sharing their outermost electrons to acquire stability by the molecule.
Why can carbon and oxygen make a triple bond to form carbon monoxide?
In the first configuration, between C and O exists a double bond, and the two atoms have zero formal charge. In the second configuration the oxygen shares a further electronic doublet and between the two atoms there is a triple bond, with the presence of formal charges.
Which elements can form double and triple bonds?
The formation of double and triple bonds is not as widespread among the atoms of the periodic table as one might expect. At least one of the atoms involved in a multiple bond is almost always C, N, or O, and in most cases both atoms are members of this trio.
Can oxygen and nitrogen form A triple bond?
Two covalent bonds form between the two oxygen atoms because oxygen requires two shared electrons to fill its outermost shell. Nitrogen atoms will form three covalent bonds (also called triple covalent) between two atoms of nitrogen because each nitrogen atom needs three electrons to fill its outermost shell.
What kind of bonds can oxygen form?
12. Oxygen atoms form 2 covalent bonds because oxygen atoms have 6 valence electrons (2 lone pairs plus 2 unpaired electrons that are shared to achieve octet).
Can oxygen form double bonds?
Like hydrogen, oxygen exists in its elemental state as a diatomic molecule. Each oxygen has 6 electrons and so need to share to more and so makes two bonds with the other oxygen. This is called a double bond.
Oxygen in Triple Bonds
Does oxygen tend to make triple bonds in resonance structures or are they considered highly unstable?
Re: Oxygen in Triple Bonds
Oxygen tends not to form triple bonds due to formal charge reasons. If oxygen starts with 6 electrons and forms a triple bond, then it has 2 lone pair electrons. Using the formal charge formula, 6 - (2+6/2) = 1. Since oxygen is so electronegative, it'll tend not to have a positive formal charge.