Why are transition metals less reactive than alkali metals?
The transition metals have the following properties in common: they form coloured compounds they are good conductors of heat and electricity they can be hammered or bent into shape easily they are less reactive than alkali metals such as sodium
What are some common reactions of transition metals?
Some transition metals react with oxygen on heating, for example: copper + oxygen → copper oxide. 2Cu(s) + O 2 (g) → 2CuO(s) Reactions with water. The group 1 elements react vigorously with ...
Which metals are the least reactive?
Which metals are included as noble often depends on preference, but a good list would be:
- ruthenium
- rhodium
- palladium
- silver
- osmium
- iridium
- platinum
- gold
- mercury
- rhenium
What transition metals are radioactive or unstable?
Polonium is a metal found in uranium ore whose isotope polonium-210 is highly radioactive, emitting tiny positively charged alpha particles. So long as polonium is kept out of the human body, it poses little danger because the alpha particles travel no more than a few centimeters and cannot pass through skin.
Are transition metals reactive or nonreactive?
Compared with the alkali metals in group 1 and the alkaline Earth metals in group 2, the transition metals are much less reactive. They don't react quickly with water or oxygen, which explains why they resist corrosion. Other properties of the transition metals are unique.
What are the properties of post-transition metals?
Post-transition metals share many similar properties including:They are solid metal under standard conditions.Like most metals they are malleable, ductile, and good conductions of heat and electricity.They have a fairly high density.More items...
Are post-transition metals radioactive?
Interesting Facts. Aluminium is the most abundant post-transition metal, and the third most abundant element on earth. The post-transition metal bismuth was considered to be the heaviest stable element until recently, before it wasdiscovered to be mildly radioactive.
What is the difference between transition metals and post-transition metals?
Physically, post-transition metals are soft (or brittle), have poor mechanical strength, and melting points lower than those of the transition metals; most also have boiling points lower than those of transition metals.
Are metalloids reactive?
The reactivity of the metalloids depends on the element with which they are reacting. For example, boron acts as a nonmetal when reacting with sodium yet as a metal when reacting with fluorine. The boiling points, melting points, and densities of the metalloids vary widely.
Is post transition a metal?
The post-transition metals, also known as the poor metals, is a group of metals on the periodic table. They are to the right of the transition metals. The Group 12 elements are sometimes included. Sometimes germanium and antimony are included, although they are normally considered metalloids.
Why are transition metals unreactive?
Transition metals are less reactive relative to I and II group due to higher ionization potential and high melting point (due to greater no of bonding electrons).
Which transition metal is the most reactive?
Caesium, the most reactive metal in the periodic table, reacts extremely violently – hence why it can't be demonstrated in a classroom! This can be compared to other common metals, such as iron and copper, which produce no reaction when dropped into water.
Is Tungsten reactive or unreactive?
non-reactiveChemical properties Tungsten is a mostly non-reactive element: it does not react with water, is immune to attack by most acids and bases, and does not react with oxygen or air at room temperature.
How many valence electrons do post-transition metals have?
2 Answers. Most transition metals have 2 valence electrons. Valence electrons are the sum total of all the electrons in the highest energy level (principal quantum number n).
Is aluminum a post-transition metal?
In the periodic table, the post-transition metals sit between the transition metals on their left, and the metalloids on their right. These metallic elements include aluminium, gallium, indium, tin, thallium, lead, and bismuth.
Is fluorine more reactive than neon?
A: Fluorine is more reactive than neon. That's because it has seven of eight possibleelectrons in its outer energy level, whereas neon already has eight electrons in this energy level.
How are silicides formed?
Silicides are usually prepared by direct fusion of the elements but coreduction of SiO 2 and a metal oxide with C or Al is sometimes used. Heats of formation are similar to those of borides and carbides but mps are substantially lower; e.g. TiC 3140°, TiB 2 2980°, TiSi 2 1540°; and TaC 3800°, TaB 2 3100°, TaSi 2 1560° C. Few silicides melt as high as 2000–2500°, and above this temperature only SiC is solid (decomp ∼2700° C).
What are the stoichiometries of silicides?
297) the formulae of metal silicides cannot be rationalized by the application of simple valency rules, and the bonding varies from essentially metallic to ionic and covalent. Observed stoichiometries include M 6 Si, M 5 Si, M 4 Si, M 15 Si 4, M 3 Si, M 5 Si 2, M 2 Si, M 5 Si 3, M 3 Si 2, MSi, M 2 Si 3, MSi 2, MSi 3 and MSi 6. Silicon, like boron, is more electropositive than carbon, and structurally the silicides are more closely related to the borides than the carbides (cf. diagonal relation, p. 27). However, the. covalent radius of Si (118 pm) is appreciably larger than for B (88 pm) and few silicides are actually isostructural with the corresponding borides. Silicides have been reported for virtually all elements in Groups 1–10 except Be, the greatest range of stoichiometries being shown by the transition metals in Groups 4–10 and uranium. No silicides are known for the metals in Groups 11–15 except Cu; most form simple eutectic mixtures, but the heaviest post-transition metals Hg, Tl, Pb and Bi are completely immiscible with molten Si.
What materials are used for gas sensors?
Similar properties have been displayed by various n- type and p- type metal oxide semiconductors as well [7,8]. The first prototype of the chemiresistive gas sensor has been developed by Seiyama et al. using ZnO thin film [9]. However, Nayoshi Taguchi patented the first gas detector in 1971 where SnO 2 film was used as a sensing material [10]. Thus, right from the beginning, the metal oxides are well-explored materials for chemiresistive gas sensing, especially extrinsic semiconducting oxides. The lattice defects/vacancies facilitate the bulk oxygen diffusion hence better sensing performance. The transition metal oxides with d0 and d10 configuration and post-transition metal oxides with d10 configuration are said to be better sensing materials [2,11]. Also, with time various electroactive polymers, 2d -materials, and carbon derivatives have also been explored.
Which group is more reactive, silanes or borides?
Silicides of groups 1 and 2 are generally much more reactive than those of the transition elements (cf. borides and carbides). Hydrogen and/or silanes are typical products; e.g.:
What is FIGURE 23?
FIGURE 23. Schematic orbital drawing of electron pathway (conduction band bottom) in covalent compound semiconductors and ionic oxide semiconductors. For color version of this figure, the reader is referred to the online version of this book.
What is the meaning of the term "Zintl phase"?
2.04.1.1 Zintl Phases – Definition. The Zintl concept is named after Eduard Zintl, a German scientist , who prepared and structurally characterized numerous binary intermetallic compounds resulting from reaction of the alkali metals with the post-transition metals or main-group metalloids.
What is the name of the rotaxanes used as rotor molecules?
Synthesis of rotaxanes (crownophanes used as rotor molecules) and their functions: 10YGK638.
How are cationic ions partially complexed?
The most successful reports to date on adjusting selectivity imply that the sample cations are partially complexed by adding a weak to moderate complexing ligand directly to the capillary electrolyte. By the partial complexation principle, only a fraction of the sample ions is converted into the complexed species which possess a lower charge and larger size than the uncomplexed metal ions. Therefore, while the free cations move along the capillary at rates proportional to their ionic mobility, the complexed metal-forms move more slowly. Evidently, the more (or less) completely a metal ion undergoes a complexation reaction or, in other words, the higher (or lower) the respective complex-formation constant, the more or less slowed will be its movement. As a result of differences in metal-ion complexing ability being greater than differences in the ionic mobility, it is possible to modulate the migration of a large number of cationic analytes over a broad range.
What is the lowest resistivity TCO?
Nowadays, tin-doped indium oxide (ITO or In 2 O 3 :Sn) is the TCO material with the lowest resistivity on a commercial scale—of the order of 1–2 × 10 −4 Ω cm [ 30, 31 ]. The resistivity of tin oxide can be as low as 5 × 10 − 4 Ω cm which depends on the dopant [ 34 ]. The industrial standard is the fluorine-doped (FTO or SnO 2 :F) [ 34 ]. ZnO doped (with gallium—GZO- or aluminum—AZO-) state-of-the-art resistivities lie in between ITO and FTO in the range of 2–4 × 10 −4 Ω cm [ 37, 39, 61 ]. A newer TCO is doped TiO 2 (the low-temperature polymorph anatase), which was introduced as a conductive transparent oxide by the mid-2000s [ 44, 45] with resistivities of around 5 × 10 −4 Ω cm for epitaxially grown Nb or Ta-doped TiO 2. In general, TCOs can be made from binary, ternary, or multicomponent oxides. The ternaries and quaternaries are formed through a combination of binary compounds. The most relevant cations can be grouped as divalent (Cd 2 + and Zn 2 + ), trivalent (Ga 3 + and In 3 + ), and tetravalent (Sn 4 + ). Examples of ternaries are Cd 2 SnO 4, CdSnO 3, Zn 2 SnO 4, CdIn 2 O 4, Zn 2 In 2 O 5, MgIn 2 O 4, or In 4 Sn 3 O 12 and quaternaries Zn 2 In 2 O 5 -MgIn 2 O 4, ZnIn 2 O 5 -In 4 Sn 3 O 12, GaInO 3 -In 4 Sn 3 O 12, or In 2 O 3 -Ga 2 O 3 -ZnO [ 14 ]. Ternary systems composed of heavy metal cations with ( n − 1) d10 ns 0 ( n ≥ 4) electronic configurations constitute attractive compounds among which Cd-Sn-O (CSO), In-Zn-O (IZO), In-Ga-Zn-O (IGZO), and Zn-Sn-O (ZTO) are the most popular for their lower cost and good thermal stabilities. A ternary used in the cadmium telluride technology is cadmium stannate (Cd 2 SnO 4 ), which can present a notable low resistivity of ~ 1–2 × 10 − 4 Ω cm [ 62, 63] forming a single-phase spinel structure whereas the resistivity of polycrystalline or amorphous zinc stannate (Zn 2 SnO 4) is higher (~ 1 × 10 − 3 Ω cm) [ 64–66 ].
What is a zinc phase?
Therefore, a Zintl phase is the product of a reaction between a group 1 (alkali metal) or group 2 (alkaline earth) elements and any post-transition metal or metalloid (i.e., from groups 13, 14, 15, or 16). Since these phases are made up of all metals, they are a subgroup of intermetallics. However, unlike intermetallic compounds, Zintl phases are typically a specific composition with very little phase width and the bonding can be understood by considering ionic bonding from the electropositive element to the more electronegative elements and then covalent (or polar covalent) bonding within the electronegative metalloids, if there are not enough electrons to form an isolated anion. Zintl recognized that there was electron transfer from the electropositive cation to the metalloids that allowed the metalloid clusters or anions to satisfy valence. The structure of the Zintl anion or polyanion should be considered based on the available electrons and the resulting electronic state. The classic example of a Zintl phase is NaTl and can be considered as an ionic formula (Na+ ) (Tl − ). In this case, Tl − is isoelectronic with group 14 and, similar to group 14 elements, forms a four-bonded diamond framework with Na + cations in interstitial sites in the structure. The idea that the polyanionic structure should be similar to an isoelectronic element was postulated by Klemm and there are many examples of this with the simple binary compounds [27]. A thermoelectric relevant phase, Mg 3 Sb 2 can be considered a Zintl phase. In the simplest interpretation, the ionic description of 3 (Mg 2 + )2 (Sb 3 −) seems like it holds [15]. However, this description does not adequately describe the bonding within the structure ( Fig. 2.5.2 ). A more complete understanding of the bonding in this structure arises from the fact that Mg can be ionic, like the rest of the alkaline earth metals although it has a fairly high electronegativity value, or covalent because of its small size giving rise to polar covalent bonds in solids, similar to Al in its bonding behavior. A recent review of Mg—group 15 containing compounds points to this dichotomy for Mg [28]. Since Mg can be considered either covalent or ionic, recognizing that it is isostructural to CaAl 2 Si 2 and considering it as MgMg 2 Sb 2 with Mg 2 + acting like Ca 2 + between the layers, and covalently bonded within layers of [Mg 2 Sb 2] 2 − provides some insight into the low thermal conductivity observed. Mg 3 Sb 2 crystallizes in the anti-La 2 O 3 structure type and compounds with this structure type can be describe as either fully ionic or fully covalent. While these different structural descriptions, CaAl 2 Si 2 vs anti-La 2 O 3, make for a useful construct depending on the application, electronic calculations suggest that the bonding of the two types of Mg, based on coordination, are similar and should be considered polar covalent [28]. Mg 3 Sb 2 is an example where the application of the ideas of Zintl is useful in either case, the fully ionic or mixed ionic- (polar)covalent structural models; but it should be recognized that the real nature of the bonding is not well described by either model. However, there are many compounds which do show formal charge transfer and follow the 8-N rule (where N is the number of valence electrons) with resultant structural features which can be considered Zintl phases.
How do cations move in a CE system?
In the most common CE system, with a fused-silica capillary and a negative polarity of its detection end, the free metal cations are expected to move by electrophoretic flow as well as by the electro-osmotic flow (EOF) working in the same direction . For some alkali-, alkaline-earth-, and a limited number of other group cations, this simple co-electro-osmotic migration mode offers rapid, efficient separations based purely on differences in ionic mobilities between cationic analytes. However, when applied to real samples, it often becomes necessary to improve the resolution by adopting special compositional electrolyte changes. For example, potassium-, sodium-, calcium-, and magnesium cations can be separated at their physiological concentrations in human serum [ 9] or animal ocular lenses [ 22 ], but only using hydroxypropylmethylcellulose to reduce the EOF.
How to detect metal ions in CE system?
In order to accomplish sensitive detection of metal ions present in the CE system in a free or partially complexed form, indirect UV absorbance detection is the most straightforward method. In this mode, the electrolyte must contain a UV-active cation with a molar absorptivity large enough to create a high background signal. Its ionic mobility also has to match those of the sample ions. Otherwise, the asymmetrical peak shapes that have generated inevitably affect the detection limits. Several aromatic bases which fulfil well these requirements, e.g., imidazole, pyridine or 4-aminopyridine, as well as copper (II) sulfate, have been found suitable for the detection of alkali-, alkaline earth-, and transition-metal ions.
What is Fig 2.5.2?
Fig. 2.5.2. (A) A view of the structure of Mg 3 Sb 2 showing the relationship to the CaAl 2 Si 2 structure type. (B) Density of states with an enlarged inset showing the contributions of the two Mg sites near the Fermi level. Both sites contribute to the top of the valence band and bottom of conduction band. In addition, calculated Crystal Orbital Hamilton Population (COHP) indicates stronger Mg2-Sb1 interactions as compared with Mg1-Sb1.
Does disproportionation occur in Cu?
Disproportionation also occurs for some transition metal cations. Disproportionation is well known in the Cu/Ag/Au column of the periodic table. Compounds that might appear to contain Au 2+, such as CsAuI 3, actually contain a mixture of Au 1+ and Au 3+, i.e., Cs 2 Au 1+ Au 3+ I 6. Similar behavior is found for AgO, which is actually Ag 1+ Ag 3+ O 2. Disproportionation has never been established for Cu 2+, but this does not eliminate the possibility that the superconductivity of cuprates is related to a tendency for disproportionation of square planar Cu 2+.
What are post transition metals?
Post transition metals are a set of metallic elements in the periodic table. It is located between the transition metals on the left and the metalloids on the right, depending on where these neighboring groups should begin at the end. There are at least five competing proposals for the elements to be included. All proposal includes Gallium (Ga), Indium (In), Tin (Sn), Thallium (Th), Lead (Pb) and Bismuth (Bi). Other names for this group are B sub group metal under p block metals. Elements 112 and 118 are post-transition metals that have not been synthesized in sufficient quantities to study their true physical and chemical properties. Some other elements are also consider as post-transition elements like Aluminum (Al), Nihonium (Nh), Flerovium (Fl), Moscovium (Mo), Livermorium (Lv).
What is Bismuth used for?
Bismuth is used in Pepto-Bismol, a medicine used to help upset stomach. Bismuth was formerly allowed to be the heaviest stable element, but lately it was discovered to be slightly radioactive.
What is the third most abundant element in the Earth's crust?
Aluminum is the third most abundant element in the Earth’s crust after oxygen and silicon. Occasionally germanium and antimony are distributed as post-transition metals rather of metalloids.
What is aluminum used for?
Aluminum (Al) is used to making in aero plane parts.
What is the third most abundant element in the Earth's crust?
Aluminum is the third most abundant element in the Earth's crust behind oxygen and silicon. Sometimes germanium and antimony are categorized as post-transition metals instead of metalloids. Gallium's melting point is only slightly above room temperature and it will melt if held in the hand.
What is Bismuth used for?
Bismuth is used in Pepto-Bismol, a drug used to help upset stomachs. Bismuth was once thought to be the heaviest stable element, but recently it was discovered to be slightly radioactive. Indium is used in electronics including flat panel displays and touch screens.
What is a post transition metal?
Post-transition, Poor, Other Metals. The post-transition metals are a group of elements in the periodic table. They are located to the right of the transition metals and to the left of the metalloids. They are also referred to as "other" metals and "poor" metals. What elements are post-transition metals?
Where does the name "thallium" come from?
The name thallium comes from the Greek word "thallos" which means " a green shoot or twig."
Is a transition metal a solid?
They have a fairly high density. In comparison to transition metals, they generally are softer and have lower melting and boiling points. Order of Abundance.
What is the relationship between electrons and cations called?
The electrostatic interactions between the electrons and cations are called metallic bonding. The electrons can move; therefore, metals have the ability to conduct electricity. Also, they are good thermal conductors. Because of the metallic bonding , metals have an ordered structure.
What type of bonding is formed between metal atoms?
The type of bond formed between metal atoms is called metallic bonding. Metals release electrons in their outer shells and these electrons are dispersed between metal cations. Therefore, they are known as a sea of delocalized electrons. The electrostatic interactions between the electrons and cations are called metallic bonding.
Why are transition metals different from other metals?
The reason for these differences is mainly due to the d electrons. Transition metals can have various oxidation states in compounds. Often, their reactivity is lower compared to other metals (for example metals in the s block). Transition metals have the ability to form colored compounds due to d-d electronic transitions.
Why are metals high melting points?
High melting points and boiling points of metals are also due to this strong metallic bonding. Moreover, metals have a higher density than water. Elements in group IA and IIA are light metals. They have some variations from the above described general features of metal.
What is transition metal?
According to the IUPAC definition, transition metal is an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell”. We normally take d block elements in the periodic table as transition metals.
What are the elements in the periodic table?
The elements in the periodic table can be divided mainly into two; as metals and nonmetals. Among these, most are metals, and there is less number of nonmetal elements in the p block.
What were the first metals discovered?
Gold and copper were the first metals to be discovered. These were used to make tools, jewelry, statues, etc. Since then, for a longer period only few other metals (17) were discovered. Now we are familiar with 86 different types of metals. Metals are very important because of their unique characteristics.
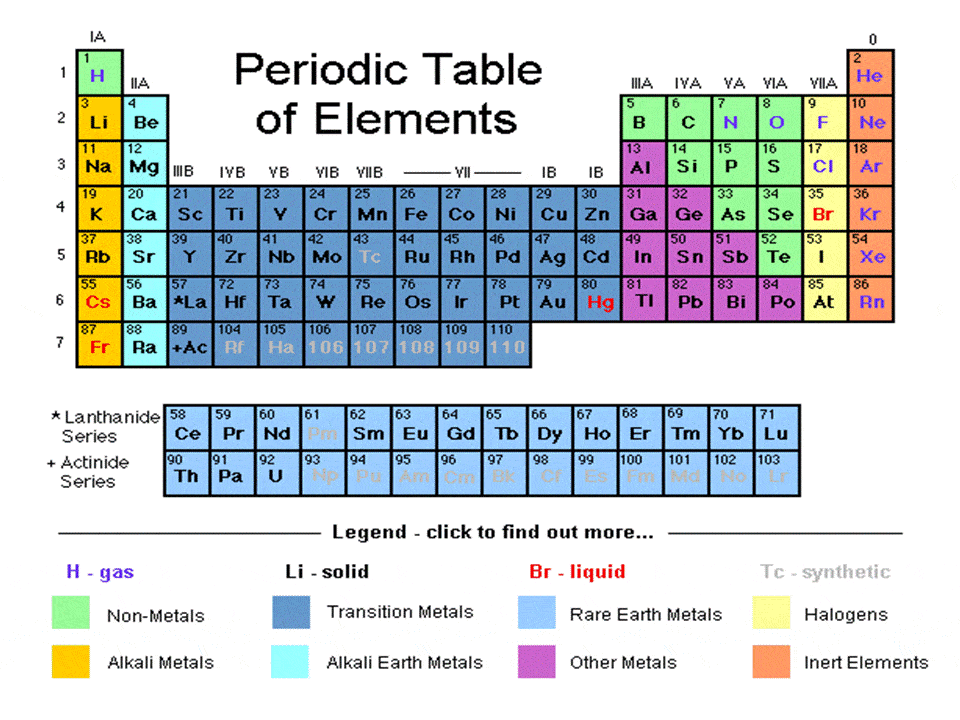
Introduction
Properties
- Physical Properties of Post Transition Metals
1. Post transition metals are soft and brittle. 2. Poor mechanical strength. 3. Have Lower boiling and melting points than of transition metals. 4. Their crystal structures tend to show covalent or directional binding effects. 5. They are generally more complex or less closely related than othe… - Chemical Properties of Post Transition Metals
1. They are characterized to varying degrees by covalent bonding tendencies. 2. They do formation of anionic species such as aluminates, stannates, and bismuthates in the case of aluminum, tin, and bismuth, respectively. 3. They can also form Zintl phases half metallic compo…
Uses of Post Transition Metals
- Aluminum (Al) is used to making in aero plane parts.
- Gallium (Ga) is used as a substitute in thermometer rather than mercury.
- Tin (Sn) is used to coat metal cans to prevent corrosion.
- Thallium (Th) was used as a rat poison and it is not used like that today because of its toxicity levels. Toxicity of this element is very high.
Some Interesting Facts Related to Post Transition
- Zinc, cadmium, and mercury are included with the post-transition metals rather than the transition metals.
- Aluminum is the third most abundant element in the Earth’s crust after oxygen and silicon. Occasionally germanium and antimony are distributed as post-transition metals rather of metalloids.
- Zinc, cadmium, and mercury are included with the post-transition metals rather than the transition metals.
- Aluminum is the third most abundant element in the Earth’s crust after oxygen and silicon. Occasionally germanium and antimony are distributed as post-transition metals rather of metalloids.
- Gallium’s melting point is only slightly above room temperature and it can melt if held in the hand for some time.
- Bismuth is used in Pepto-Bismol, a medicine used to help upset stomach. Bismuth was formerly allowed to be the heaviest stable element, but lately it was discovered to be slightly radioactive.